Fishes of Canada's National Capital Region
Brian W. Coad
Canadian Museum of Nature,
Ottawa, Ontario, Canada
Contents
Updates (significant new information not in the main text)
Species Accounts (species descriptions, biology, figures, maps)
Checklist (official scientific, English and French names)
Keys (for identification)
© Brian W. Coad (www.briancoad.com)
This work is a guide to the fishes found in the National Capital Region (NCR) of Canada, a region encompassed by a circle of 50 km radius centred on the Peace Tower of the Parliament Buildings in Ottawa, extending into Ontario and Québec. An earlier work by Coad and McAllister (1975) is dated and required a revision. The book treated 75 species while this work covers 84 species and, as the updates show, species new to the NCR are still being recorded.
Most illustrations are of fishes and localities from within the NCR although some are from other sources, as noted when a cursor is placed over them. Maps can be clicked on to show a larger version with caption details.
Additions to the main text of this website terminated in 2010. However, some notes and updates will be added here if they are deemed significant. Descriptions and keys to species new to the NCR and not in this text can be found in Scott and Crossman (1973) and Coad et al. (1995).
• A tilapia, believed to be the Blue Tilapia (Oreochromis aureus (Steindachner, 1864)), has been found in a stormwater retention pond in Ottawa (Nicholas Mandrak, pers. comm., 23 June 2010). It is probably a released aquarium fish and does not probably represent an established population.
• The Central Stoneroller/Roule-caillou (Campostoma anomalum (Rafinesque, 1820), Cyprinidae) has been collected in the Jock River at Bleeks Road (Brian Bezaire, pers. comm., 9 June 2010), a new record for the NCR. Stonerollers have been seen in Ottawa bait shops for many years.
• The Bridle Shiner/Méné d'herbe (Notropis bifrenatus (Cope, 1867), Cyprinidae) has been captured in the Rideau River at localities near James Island (Jason M. Barnucz and Brian Bezaire, pers. comm., 9 June 2010), a new record for the NCR.
• Photographs of a fish caught in April 2010 at Victoria Island in downtown Ottawa (courtesy of Tim Haxton, 22 April 2010) appear to be a White Bass/Bar Blanc (Morone chrysops (Rafinesque, 1820), Moronidae). This new species record for the NCR needs confirmation with voucher specimens.



Central Stoneroller, Bridle Shiner and White Bass
• Lampreys, as yet unidentified, have been caught in the Bearbrook watershed east of Ottawa (North Indian Creek and an unnamed tributary of the Bearbrook) (Josh Mansell, South Nation Conservation, 31 March 2011).
Scientific Name: Acipenseridae Amiidae Anguillidae Atherinopsidae Catostomidae Centrarchidae Clupeidae Cottidae Cyprinidae Esocidae Fundulidae Gadidae Gasterosteidae Hiodontidae Ictaluridae Lepisosteidae Osmeridae Percidae Percopsidae Petromyzontidae Salmonidae Sciaenidae Umbridae
English Name: Bowfins Carps and Minnows Cods Drums and Croakers Freshwater Eels Gars Herrings Lampreys Mooneyes Mudminnows New World Silversides North American Catfishes Perches Pikes Sculpins Smelts Sticklebacks Sturgeons Suckers Sunfishes Topminnows Trout-perches Trouts and Salmons
French Name: Achigans et Crapets Anguilles d'eau douce Barbottes et Barbues Brochets Carpes et Ménés Catostomes Chabots Éperlans Épinoches Esturgeons Fondules Harengs Lamproies Laquaiches Lépisostés Morues Omiscos Perches et Dards Poissons d'argent Poissons-castors Tambours Truites et Saumons Umbres
The Species Accounts are listed above alphabetically by families under scientific name, English common name and French common name. In the text below, the families and their included species are arranged taxonomically, as is traditional, and the links above help locate the families.
Species Accounts are preceded by a family account where general characters and biology shared by all members of the family are explained to avoid repetition under each species. Families with only a single species in the National Capital Region (NCR) may have only a few comments here as characters and biology are subsumed in the Species Account. Further information on the fishes can be found in Encyclopedia of Canadian Fishes (Coad et al., 1995). Some of the NCR fishes are also found in marine waters where their biology can be quite different. The higher relationships of the fishes and further information on their relatives in other parts of Canada are also dealt with in that work.
Layout of Species Accounts
Species Accounts are comprised of a line drawing, colour drawings, colour photographs of fish and fish habitats where available, a spot distribution map and a text with taxonomy, key identification characters, a description of the species including colour and size, general distribution, origin, habitat, biology and importance. The species are arranged by scientific name within each family so related fishes are next to each other.
Maps can be clicked on to show a larger version. Colour drawings and photographs have data available by running a cursor over them.
Unattributed statements in all parts of the Species Accounts are based on a survey of the general literature for the species and may not always apply in the NCR. For example, Lake Sturgeon are described as occurring in lakes (as the common name indicates) but in the NCR they are found only in the Ottawa River. Referenced items or items which specifically mention areas within or near the NCR carry local information.
The line drawing shows characters which may not be evident on a photograph, colour photographs show natural colours and variations as well as size and shape, and habitat photographs give a pictorial summary of habitats.
The spot map shows capture localities based on preserved specimens in the Canadian Museum of Nature, Ottawa and other museums (susceptible to re-examination and correction), and on literature records, records from websites, and anecdotal accounts judged to be accurate reports (but not susceptible to re-examination other than further field work). Coad (1985a) gives a report on an error of distribution, for example, and many others have been analysed for this study. Certain records of species have been rejected as there are no voucher specimens available for confirmation and they have not been reported from the NCR in other literature records. Efforts to collect unusual literature records in the field were made without success: they are most probably misidentifications.
All localities are stored in a database. The commoner species with a wide tolerance of environmental variables and easily capturable are much more widely distributed than these spots might suggest while the rarer species, with few spots, are probably a more accurate reflection of distribution. Phelps et al. (2000), for example, captured over 6900 fishes in less than two months on the Rideau River but only one of these was a Freshwater Drum. Records also reflect ease of capture, itself a reflection of gear used, time of use, effort and simple luck. Sport fishes may have fewer localities since they may have restrictions on licensed collecting or localities may be kept secret to protect stocks. Some rivers or lakes have been intensively surveyed while others have been sampled only sporadically and/or at widely separated localities.
A map showing all localities sampled can be accessed here.
Taxonomy gives some other common names, reviews changes in scientific names, reports hybrids and covers other systematic and taxonomic problems.
Key identification characters separate a species from others within its family and from other fishes in the NCR. These key characters are only applicable within the NCR. However some species have obvious and unique characters which separate them from all other fishes. Some fishes are more subtly distinguished and characters in combination may have to be used or body proportions. Body proportions are often not as clearly defining as unique structures or countable characters, varying with growth, maturity or even fullness of the gut. Countable characters may overlap in ranges. The separate section of Keys should also be consulted.
The species description is based on specimens from within the National Capital Region and on others from elsewhere in Canada and North America. The range for meristic characters is given for the species as a whole. Counts based purely on local fish or on a few specimens may give a misleading impression of the potential range should more specimens be examined.
Colour is based on specimens when alive but may also have details only visible on preserved fishes.
Size gives length, usually total length, but sometimes standard or fork length.Where the type of length is not indicated this is because it was not given in the source. Weights are not always known for the smaller species. Record fishes caught by angling may also be cited. Since fish grow continuously, although much more slowly with age, there is no definitive maximum size and larger specimens may always be caught.
Origin gives the refugium from which the species entered the NCR as the there were no fishes here until the glaciers retreated after the last ice age. A history of the area and detailed routes and timing of entry are given in McAllister and Coad (1975), Rubec (1975a), Bailey and Smith (1981), Legendre and Legendre (1984) and Mandrak and Crossman (1992).
Habitat gives details of the environment in which the species is found.
Distribution places the species in context for North America and the world and some features of distribution in the NCR may be discussed.
Biology sections (Age and Growth, Food and Reproduction) are based firstly on that known for the species throughout its range with notes from local fishes where available. Few local fish populations have been studied intensively. Time of reproduction, for example, will vary with local conditions such as temperature but is seasonally the same for the species across Canada. Details of diet will also vary depending on availability of food species in the habitat type or region of the country but will be generally similar. Growth depends on food supply, competition, and various environmental factors.
Importance gives details of the significance of the species commercially, recreationally and biologically. Some species have "none" under this heading, indicating that they are not significant commercially or recreationally and that their biology is poorly known, although any species has importance in its ecosystem. Dymond (1939) provides a review and accessible summary of fisheries in the general vicinity of the NCR in the late nineteenth and early twentieth century while noting fisheries data is recorded from different areas at different dates making comparisons difficult. In 1898 over 700,000 lbs (317,518 kg) of fish were taken from the Ottawa region (which includes some waters outside the NCR as defined here), of which 70,300 lbs (31,888 kg) were bass (presumably Smallmouth Bass) and 37,750 lbs (17,123 kg) Muskellunge. This level of fishing, coupled with pollution (particularly sawdust), steadily depleted stocks.
Petromyzontidae - Lampreys - Lamproies
Lampreys are found in cooler waters of the northern and southern hemispheres. There are 41 species with 11 recorded from Canadian freshwaters and along all three coasts. There are 4 species in the NCR.
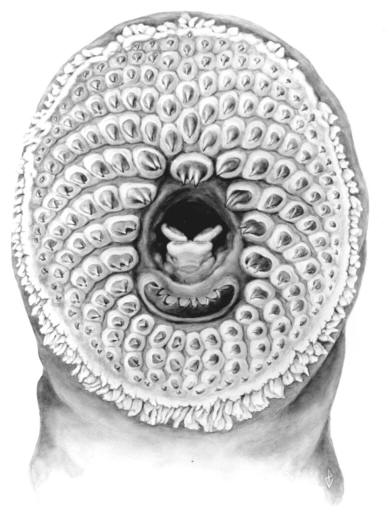
Lampreys are jawless fishes, lacking bone in the skeleton and having 7 pairs of pore-like gill openings. The eel-like body has no pectoral or pelvic fins. There are 1 or 2 dorsal fins and a caudal fin. An anal fin-like fold develops in spawning females. Eyes are large. The mouth is a suctorial disc armed with rows of horny teeth. There are also teeth on the tongue. The median nostril, or nasohypophyseal opening, is not connected to the mouth. There is a light-sensitive pineal organ or "third eye" behind the nostril. The skin is covered in mucus which is poisonous to fishes and humans. Lampreys are edible if the mucus is cleaned off. Lampreys have a body form similar to the more familiar but unrelated eels (Anguillidae). Occasionally there are references in articles to lamprey-eels, but there is no such fish; there are lampreys and there are eels, quite distinctive organisms.
Their origins lie at least 300 million years in the past. Their tooth arrangement is used in classification and identification along with the number of myomeres (muscle blocks along the body). Both tooth counts and the number of cusps are used in particular those on the supraoral lamina (bar above the "mouth", the oesophageal opening), the infraoral lamina (bar below the "mouth") and the row of teeth on both sides of the "mouth". There are various series of smaller teeth and of course teeth on the tongue. Larval lampreys lack teeth and are particularly difficult to identify and their determination often requires specialist knowledge. Characters for the larvae include counts of myomeres and pigmentation patterns.
Lampreys have an unusual life cycle. Adults die after spawning and the eggs develop into a larva, known as an ammocoete, which lacks teeth, has an oral hood, eyes covered by skin, a light-sensitive area near the tail, and is a filter-feeder while buried in mud and silt. Fleshy tentacles in the oral hood are used to extract minute organisms from the water, such as algae (desmids and diatoms) and protozoans. After several years (up to 19 but usually 7 or less), the ammocoete transforms into an adult with enlarged eyes, teeth, a different colour and pronounced dorsal fins. The body shrinks during this metamorphosis and adults are only larger than ammocoetes if they feed. The adult may be a parasite on other fishes and marine mammals, or non-feeding. Individuals of a species may or may not be parasitic and different species may be parasitic or non-parasitic. The non-parasitic species are believed to have evolved from a parasitic species so there tends to be closely related parasitic/non-parasitic species pairs.
Parasitic adults feed mostly on other fishes, attaching to their bodies by suction and using their toothed tongue to rasp through the skin and scales to take blood and tissue fragments. Prey is detected by sight but some lampreys attach to hosts during the night. Perhaps this reduces their own predation risks and enables them to approach their quiescent hosts more easily. Lampreys tend to select larger fish as these survive longer and ensure a good food supply. The flow of blood is aided by an anti-coagulant in lamprey saliva called lamphedrin which also serves to break down muscle tissue. Large, anadromous lampreys are usually attached ventrally near the pectoral fins while small, freshwater species, such as the Chestnut Lamprey and the Silver Lamprey, are usually attached dorsally. Dorsal attachment reduces abrasion of the lamprey in shallow water. Ventral attachment results in greater food intake for the lamprey. Lamprey attacks leave a characteristic round scar and can be a major problem for commercial fisheries by damaging food species and leaving them too unsightly to market. The attack may weaken or even kill the host. Weakened fishes are more prone to diseases and the wound provides an easy path of entry for them. Even fishes with heavy scales like Gar Family members are attacked. A single 16 kg Lake Sturgeon has been recorded with 61 Silver Lampreys parasitising it, although it was estimated that this would not kill the host by draining its blood.
Lampreys may move into or up streams to spawn. The scientific name of the family means "stone sucker" and the adult mouth is used to hold or suck onto stones as well as on prey. This suction enables the lamprey to maintain position in fast-flowing streams when spawning and even to climb over rapids and small waterfalls. Usually spawning occurs in shallow water with a moderate current, a bottom of gravel and nearby sand and silt for the ammocoetes to live in. Either or both sexes build a nest by moving gravel around with their sucking mouths and by thrashing their bodies. A shallow depression is formed, about 0.5-1 metre long. Spawning often occurs in groups and several males may attach to a female with the sucking disc. The process takes several days as only a few white to yellow eggs are laid at a time. The eggs are adhesive.
Adult lampreys are usually caught when attached to a host or when spawning. Electro-shocking will force ammocoetes out of their u-shaped burrows to the surface and immobilise adults. They sometimes attach to boats and occasionally to swimmers when their skin is cool but are easily removed, perhaps because nobody has left a lamprey on their skin long enough to see if the tongue starts rasping flesh!
Lampreys have been used for food by various native peoples in Canada and are popular in Japan. They have been considered a delicacy and can be smoked, set in aspic or cooked in a variety of ways. Henry I of England is said to have died of a "surfeit of lampreys".
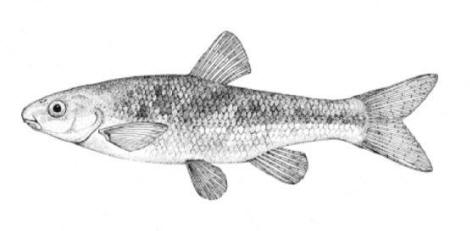
Chestnut Lamprey / Lamproie brune
Ichthyomyzon castaneus Girard, 1858
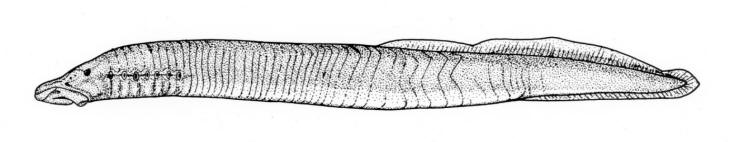
Taxonomy
Other common names include Western, Northern, Silver, and Brown Lamprey, Hitchhiker, Seven-eyed Cat and Bloodsucker. Ichthyomyzon is from the Greek ichthys for "fish" and myzon for "sucking" and castaneus is Latin for "chestnut-coloured".
Key Characters
This lamprey is distinguished by having a single, notched dorsal fin, by having 1 or more lateral disc teeth with 2 cusps (1-10 bicuspid circumorals, usually 6).
Description
There are 47-57 trunk myomeres, usually 51-54. Teeth are sharp, strong and curved. The band of teeth below the mouth is a broad, curved bar with 6-11 tooth cusps. There are 4 pairs of inner lateral teeth, usually bicuspid and sometimes tricuspid.
Colour
Adults are dark grey to olive, or yellow-brown, sometimes mottled, occasionally a chestnut colour which gives them their name. The lateral line organs are black in adults, though only weakly in young adults. They become blue-black when spawning. Ammocoetes are overall lighter in colour. Ammocoetes are paler and have no pigment, or are only weakly pigmented, on the lateral line organs.
Size
Attains 38.0 cm.
Found in central North America from southeastern Saskatchewan, west-central Manitoba and eastern Lake Huron tributaries of Ontario south to the Gulf of Mexico centred on the Mississippi-Missouri basin.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Found mostly in medium-sized streams although adults may be found in large rivers and dams. Ammocoetes prefer vegetated areas with current unlike other species.
Age and Growth
Ammocoete life span is unknown, but is presumed to be 5-7 years with a maximum age for the species of about 8 years.
Food
Transformed adults do not feed over their first, and one subsequent, winter and feed most heavily from April to October. They spawn and die the following summer. This species has been caught attached to a Lake Sturgeon or a Northern Pike in Brewery Creek in the NCR (the data were uncertain), most probably the latter given the locality (Renaud and de Ville, 2000). The eggs of this lamprey are eaten by darters, minnows and crayfishes.
Reproduction
Peak spawning is in early to mid-June at 15.6-22.2°C in Michigan and takes place at night in small to large groups (up to 50). A female attaches to a stone and begins rapid quivering motions. A male attaches to the head of the female and wraps its tail around her body. Up to 5 lampreys can attach, each to the head of the one before it. Up to 50 lampreys can be found in a single nest as nests tend to merge during excavation. The nest sites are in small streams. A single nest can be 2.8 m long and 1 m wide. The spawning continues through the night but by the afternoon of the following day, lampreys have left the nest. A large female had 42,000 eggs. Eggs are elliptical, 0.64 mm by 0.56 mm.
Importance
This lamprey was accorded a status of "vulnerable", now "Special Concern", in 1991 by the Committee on the Status of Endangered Wildlife in Canada. It is not as economically significant as the Silver Lamprey because of its habitat, size, abundance and distribution in Canada. Elsewhere it is known to attack Brook Trout and can stay attached for over 18 days, killing the host. Trout destruction has been reported as reaching 23.5 kg/ha or about one-third of the trout available to anglers. They are a favoured food of Burbot and trouts as well as other fishes.
Northern Brook Lamprey / Lamproie du nord
Ichthyomyzon fossor Reighard and Cummins, 1916
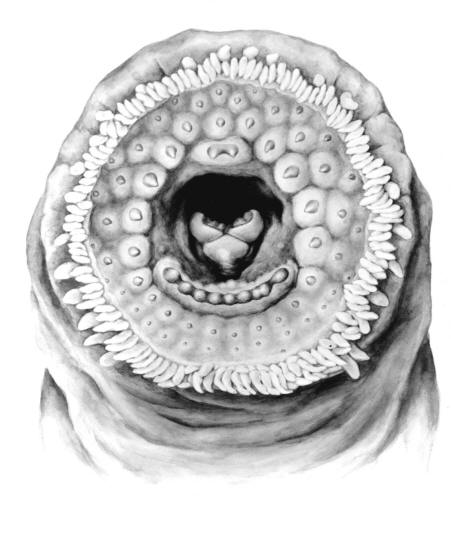
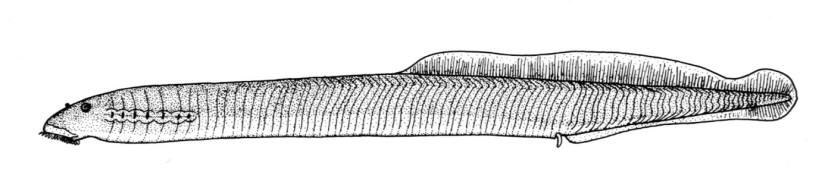
Taxonomy
Other common names include Michigan Brook Lamprey and Blood Sucker.
Key Characters
This species is distinguished from other Canadian lampreys by having a single dorsal fin, teeth along the side of the mouth with 1 cusp, 2 knob-like and blunt cusps on the bar above the mouth, 6-11 knob-like and blunt cusps on the bar below the mouth, and lateral lines organs unpigmented.
Description
Trunk myomeres number 47-58, usually 51-54. The sucking disc is narrower than the body.
Colour
Adults are dark slate grey to brown above, pale grey, silvery or white on the belly. The area under the gill pores may be orange. Fins are grey to yellow or brown. The eye is bluish. Lateral line organs lack black colouration. In ammocoetes, the caudal fin and head are weakly pigmented.
Size
Attains 28.2 cm and 9.9 g.
Found in the Hudson Bay drainage of Manitoba, the Great Lakes basin of Ontario and the upper St. Lawrence River basin in Québec south to Kentucky and Missouri. The presence of this species in the NCR is uncertain (Coad, 1986b). Adults are not easily caught with nets because of their elongate shape and, being non-parasitic, cannot be found attached to fish. Ammocoetes can be caught in mud or sand by electroshocking but are very difficult to identify and distinguish from the Silver Lamprey. Spawning adults would be readily identifiable but attempts to catch them have failed as spawning takes place in early spring when water levels are high, conditions are difficult of access in flooded areas of uncertain depth. Lanteigne (1981; 1992) mapped the Northern Brook Lamprey at Ottawa but Rohde and Lanteigne in Lee et al., (1980) did not.
Origin
This species entered the NCR from a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
This is a non-parasitic species found in warmer streams and smaller rivers than the American Brook Lamprey or along the margins of larger rivers. It is reported as common in turbid streams. Ammocoetes prefer a soft bottom over firm sand but not the extremely soft mud of backwaters. They are most numerous at 15-61 cm depths. They move only if their habitat is disturbed or food becomes short. This movement takes place mostly at night when predators are less active. Low fertility and relatively low mobility as a non-parasitic species make it vulnerable to natural and man-made habitat changes.
Age and Growth
Ammocoetes live 5-7 years and may "rest" for a year without feeding before transformation to the adult in August to September. Maximum life span is about 8 years.
Food
Ammocoetes filter feed on desmids, diatoms and protozoans. Food may also be taken from the sediment. The gut degenerates at the beginning of transformation from ammocoete to adult and for a period of 8-9 months no food is taken. Various predatory fish will take ammocoetes and spawning adults on nests are most vulnerable.
Reproduction
Spawning occurs from late May to mid-June at 12.8-23.3°C in Michigan without a migration. The nest is constructed among large stones or gravel to create a cavity. Unusual vertical body movements, and transport of gravel using the sucking disc, excavate a 10 cm long nest. Up to about 2,000 eggs of 1.0-1.2 mm diameter are produced and adhere to silt-free sand. Incubation takes 9 days at 18°C. The post-spawning period lasts only a few days and then all the spawning adults die.
Importance
Ammocoetes have been sold as bait in Quebec but this now prohibited. This species was accorded the status of "Special Concern" in 1991 by the Committee on the Status of Endangered Wildlife in Canada (Lanteigne, 1992; COSEWIC, 2002).
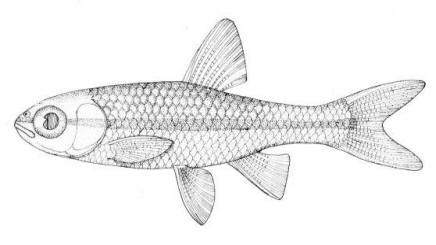
Silver Lamprey / Lamproie argentée
Ichthyomyzon unicuspis Hubbs and Trautman, 1937
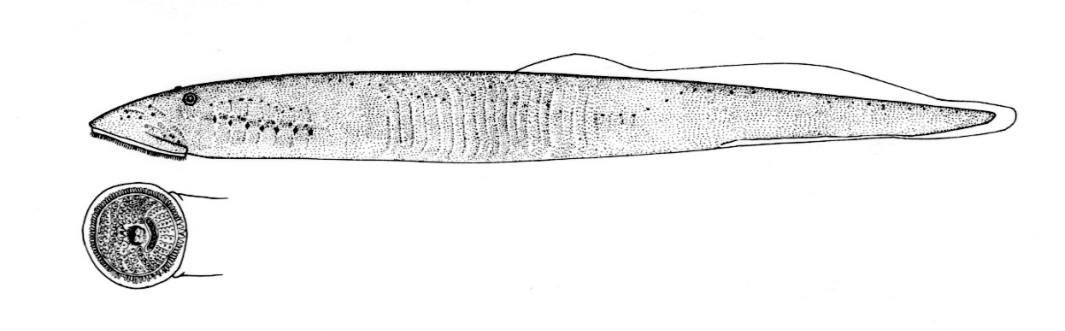


Taxonomy
Other common names include Northern Lamprey and Brook Lamprey. The Northern Brook Lamprey is its non-parasitic relative.
Key Characters
This species is distinguished by having a single dorsal fin, all teeth along the sides of the mouth with 1 cusp, (0-2 bicuspid circumorals, usually none), a sharp bicuspid tooth on the bar above the mouth, and 5-11 sharp, triangular cusps on the bar below the mouth.
Description
Teeth are sharp and yellow. Trunk myomeres number 47-57, usually 49-52. The sucking disc is wider than the body.
Colour
Adults are brown, blue-grey or bluish with silvery overtones and with a blue-grey or silver belly. Lateral line organs are black in specimens greater than 150 mm length. Adults become darker as the spawning season approaches and are blue-back near the end of spawning. Ammocoetes are pale although the caudal fin and head are strongly pigmented.
Size
Attains 38.1 cm.
Found from Manitoba, through southern Ontario around the Great Lakes to western Québec and as far south rarely to Mississippi. In the NCR this species is known only from the Ottawa River and mouths of tributary rivers. Small (1883) reports Icthyomyzon Argenteus (sic) (= I. unicuspis) from the "Rideau, Gatineau, the Lievres, and streams running into these rivers" but this has not been borne out by field work since then
Origin
Silver Lampreys entered the NCR from a Mississippian refugium.
Habitat
This is a parasitic species found in the larger rivers and lakes. Ammocoetes live in burrows in soft bottoms.
Age and Growth
Ammocoetes live 4-7 years before beginning transformation in late fall. Adults live 12-20 months and may migrate to a lake to feed. Females grow faster than males and attain a larger size.
Food
Ammocoetes filter-feed phytoplankton and other small organisms from the water. Adults are parasites on Lake Sturgeons, Brown Bullheads, American Eels, White Suckers, Silver Redhorses and Shorthead Redhorses (McAllister and Coad, 1975), on Northern Pike (attached to the inside of the branchial cavity of one pike, C. B. Renaud, pers. comm., 2002) and on Muskellunge (Renaud, 2003) in the NCR. Marks per host based on 15 Muskellunge from the Ottawa River from Ottawa to Hawkesbury varied between 1 and 31, with a mean of 10.6. 84.6% of the Muskellunge had marks on the dorsal surface, 46.2% laterally and 15.4% ventrally. The position of attachment was thought to be a consequence of the predatory behaviour of the Muskellunge, lying in concealment with the belly less exposed than in pelagic species, and perhaps to avoid detachment or injury by abrasion on the substrate. The wounds on the Muskellunge suggest blood feeding as they were shallow, rather than flesh feeding. The lampreys fed actively from at least 21 June to 30 October when captures with fresh marks were made.
Reproduction
Both sexes construct the nest in gravel in running water. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 13 May to 23 June at 12.5-19ºC. Spawning occurs in May-June, peaking in early June, at 12.8-22.8°C in Michigan. Up to 65,000 eggs of 1.0 mm diameter may be produced. The eggs hatch after 7-10 days, depending on water temperature.
Importance
The ammocoetes are used as bait for sport fish.
American Brook Lamprey / Lamproie de l'est
Lampetra appendix (DeKay, 1842)
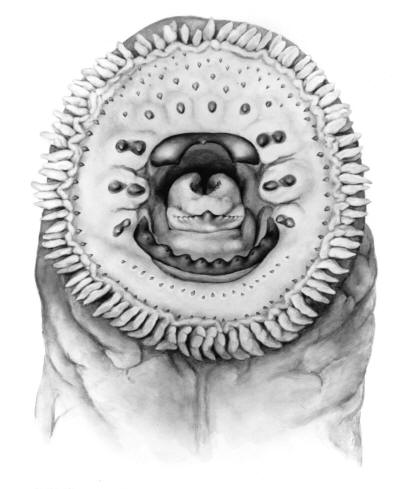
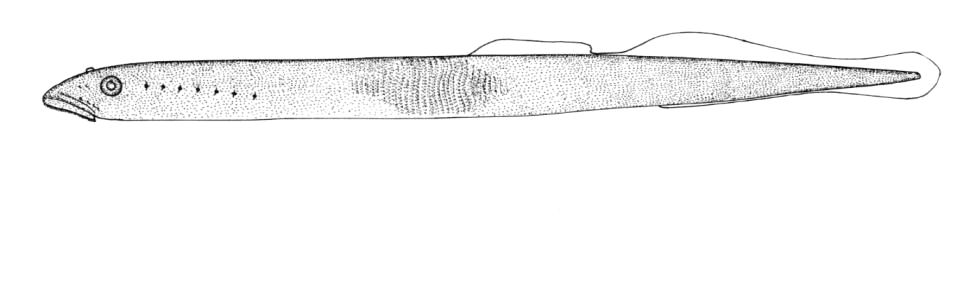
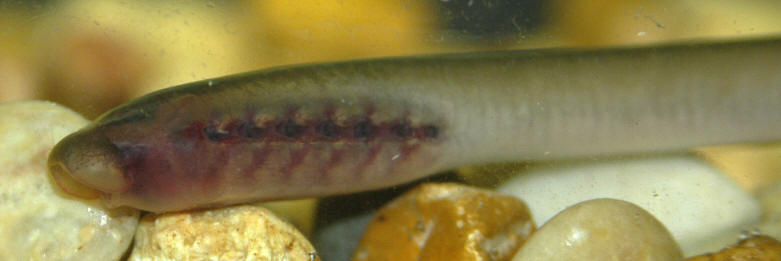
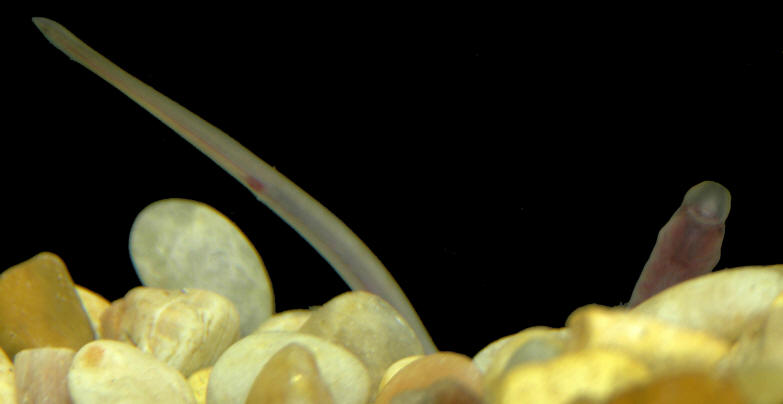
Taxonomy
Also called Brook Lamprey or Small Black Brook Lamprey. Scientific names used in other works that may be this species are Lampetra lamottenii Le Sueur, 1827 and Lampetra wilderi Jordan and Evermann, 1896. The former name has priority but there is some confusion over Le Sueur's application of this name and L. appendix is the next available name. Lampetra is from the Mediaeval Latin for "lamprey" and appendix is Latin for "appendage", probably referring to the prominent urogenital papilla of adult males.
Key Characters
This species is distinguished by having 2 dorsal fins, the bar above the mouth with 2-3 pointed cusps, and teeth along each side of the mouth bicuspid and pointed.
Description
Trunk myomeres number 63-74. The bar below the mouth has 6-10 cusps. Teeth are generally blunt and not as sharp as in the parasitic Silver Lamprey. Shrinkage of adults over winter from non-feeding almost closes the gap between the dorsal fins.
Colour
Adults are blue-grey when spawning with orange tinges on the head, back, tail and fins. Otherwise brown or lead-grey is the overall colour, the belly is white to light grey or silvery and clearly set off from the flanks. Fins are yellowish with the caudal fin darkest near the base, becoming lighter towards the margin. Ammocoetes are pale brown and have a whitish band above the branchial openings.
Size
Attains 31.7 cm (perhaps 35 cm) but these were "giants" which may have fed parasitically. Usually up to about 21.7 cm.
This species is found from the Great Lakes basin of Ontario and the Upper St. Lawrence River basin in Québec southwards to Virginia, Tennessee and Missouri.
Origin
This lamprey entered the NCR from possibly a Mississippian refugium or Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
This is a non-parasitic species found in cooler (9-12°C) small streams and rivers than the Northern Brook Lamprey. It is sensitive to environmental change and prefers water that is clean and free of silt. Around Kettle Island and Upper and Lower Duck Islands in the large Ottawa River it is found unusually in sandy areas at 13-25°C (Lanteigne et al., 1981). It does not migrate.
Age and Growth
Ammocoetes live 4-6 years (perhaps 7.5 years) and transformation begins in the fall and continues over winter. The adult shrinks without food from parasitism.
Food
Adults do not usually feed and ammocoetes filter fine particles from the water.
Reproduction
Spawning occurs in mid-May to early June in Québec at 17°C and in Ontario in April-May. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 3 May to 20 May at 9-14ºC. In Minnesota it occurs during late April and early May at 8.7-15.5°C. In Delaware spawning occurs in March-April at 6.8-12.0°C and in Michigan at 6.7-20.6°C in early May. Nests up to 30 cm long are built to a depth of 4 cm below the stream bottom. Current velocity in a Minnesota study was 14 cm s-1. The male begins construction and the oval to circular nests are in gravel and cobble between larger rocks or just upstream of riffles. Up to 25 lampreys may spawn in one nest with 5 times as many males present as females. The male uses his sucker to attach to the female's head, arching his body to bring his cloacal region close to the female's. Nests tend to be larger in deeper water, slower current and where larger spawning groups are found. Up to 5185 pale yellow to light green eggs about 1.2 mm in diameter are produced by each female. The eggs hatch in 9 days at 68°F.
Importance
Ammocoetes have been sold as bait for sport fish in Québec but this is no longer permitted.
Acipenseridae - Sturgeons - Esturgeons
Sturgeons are found in fresh and coastal waters around the Northern Hemisphere. There are 24 species with 5 occurring in Canada and 1 in the NCR. This family contains the largest freshwater fishes and life span is reported to exceed 150 years although fish of great size are difficult to age accurately. Some species live entirely in fresh water while others are anadromous, spending some time in the sea but returning to fresh water to spawn. The NCR species spends all its life in fresh water. Sturgeon roe or eggs are known as caviar and form an expensive delicacy. The flesh is also eaten, and is tasty when smoked. The swimbladders of sturgeons have been converted to isinglass, a transparent gelatin used in a variety of products including as a wine and beer clarifier and in jams and jellies. The total Canadian catch of sturgeons in 1988 was 53 tonnes. Their migratory habits have made them victims of pollution and hydroelectric schemes and sturgeons are no longer as large nor as numerous as in the past. Slow growth makes them susceptible to overfishing. Fossils extend back 100 million years and related but extinct families to 310 million years ago.
Lake Sturgeon / Esturgeon jaune
Acipenser fulvescens Rafinesque, 1817



Taxonomy
Other common name include Freshwater, Common, Great Lakes, Red, Ruddy, Black, Rock, Stone, Dogface, Shellback or Bony Sturgeon, Smoothback, Rubber Nose, Esturgeon de lac and Camus; or for young - Escargot, Maillé and Charbonnier. Acipenser is from the Latin for "sturgeon" and fulvescens means "brownish".
Key CharactersThis species is unique in having 5 rows of bony scutes or plates along the body, (1 dorsal, 2 lateral and 2 ventrolateral), 4 barbels in front of the transverse mouth under an elongate snout, no teeth in adults, and an upturned caudal fin skeleton (heterocercal) such that the upper tail lobe is larger than the lower.
Description
Dorsal fin rays 35-40, anal fin rays 25-30, dorsal plates 8-17, lateral plates 29-43, ventral plates 6-12, 1 large plate between the caudal fin and the anal fin in addition to the fulcrum (a flat bony plate), and 25-40 gill rakers. The scutes may almost disappear in old sturgeons but are sharp and obvious in young. The skeleton is cartilaginous, there is a spiracle and a spiral intestinal valve.
Colour
Back and upper flank dark brown, olive-green or grey and belly white to yellow-white. Flanks may be reddish. Fins are dark brown or grey. Body cavity organs are black but the peritoneum is silvery and only slightly pigmented. Specimens smaller than 30 cm have 2 black blotches on the upper snout, a black blotch between the dorsal and lateral plates above the pectoral fin base and another similarly positioned below the dorsal fin, and smaller spots on much of the rest of the head and body. Lower parts of the body are greenish.
Size
Reaches an estimated 312.0 cm and 184.57 kg, the largest freshwater fish in North America. The world, all-tackle angling record wa recognised as a 41.84 kg fish caught in the Kettle River, Minnesota in 1986 although much larger fish have been caught on rod and line. The Ontario record as of the year 2000 from Georgian Bay weighed 76.2 kg and is the International Game Fish Association Record (1999). The fish was caught in 1982. Newspaper records of large sturgeons caught in the Ottawa River are reprinted in Szabo (2004) and listed here. One fish snagged in Lac Deschênes had a sleigh harness entangled around its gills, apparently lost ten years before when a horse fell through the ice (Pembroke Observer, 7 June 1901, p. 1). Catches in the Ottawa River include a 5' 3", 85 lb one caught between Aylmer and Ottawa (Toronto Star, 22 May 1908, p. 1), a 5'4", 100 lb one caught illegally - the fisherman was fined (Globe and Mail, 2 November 1921), a 5'6" (1.65 m), 175 lb (79.5 kg) one from Lac Deschênes that had to be towed 2 miles to shore to be landed (Globe and Mail, 17 May 1927, p. 1; Harkness and Dymond (1961); McAllister and Coad (1975)), a 217 lb (98.5 kg) one from near Montebello that either bit or swiped the angler on landing (Globe and Mail, 26 March 1931, p 1; Toronto Star, 26 March 1931, p. 6; Butler, 2006; Harkness and Dymond (1961); Egan (2000)), a 5', 75 lb one caught with a minnow net (Toronto Star, 3 July 1939, p. 26), a 4' one caught by its tail from a ferry between Rockliffe and Gatineau Point (Toronto Star, 8 June 1949, p. 48), a 60 lb one that leaped out of the water at Rockliffe boathouse (Toronto Star, 19 August 1949, p. 1), a 3', 15 lb one that jumped into a boat - it apparently was trying to rub leeches of its body on the side of the boat (Toronto Star, 2 July 1953, p. 10), a 51.5", 45 lb one near Orleans (Globe and Mail, 23 June 1962, p. 4), and a 5'10" (1.8 m), 107 lb (48.6 kg) one from the Deschênes Rapids (Ottawa Citizen, 7 July 1972, p. 3; Cote (1972); Leggett (1975)).
Found from western Québec including James Bay, all of Ontario and most of Manitoba and westward in the North Saskatchewan River to Alberta. South to Alabama, northern Mississippi and Arkansas west of the Appalachian Mountains. In the NCR it is restricted to the Ottawa River and mouths of tributaries, with more captures below Ottawa than above in the NCR (see also Haxton(2008) for additional map points). .
Origin
This species colonised the NCR from a Mississippian refugium (Guénette et al., 1993; Ferguson and Duckworth, 1997).
Habitat
Lake Sturgeon are found in shallow areas of lakes and large rivers at about 4-9 m although they may descend to 43 m. These shallow areas are the highly productive shoals where food items are most available. Mostly the bottom is mud. Older fish tend to be in deeper waters and all age groups move away from shallower waters in summer to return in fall, presumably in response to temperature and oxygen changes. They are not usually found in water over 23.8°C and prefer waters at 15-17°C. Occasionally sturgeon are reported to jump and can end up in boats (Harkness and Dymond, 1961; Egan, 2000; 2003). A 50 lb (22.7 kg) sturgeon 58 inches (1.47 m) long leaped out of the water and landed in a rowboat crewed by two girls on the Ottawa River. Sturgeon are quiet tough and can survive several hours out of water and are relatively easy to tag, spine clip and weigh and measure in mark-recapture studies when water and air temperatures are cool.
Kerr et al. (2010) review Lake Sturgeon habitat requirements and the effects of habitat changes such as dams. A barrier-free distance of 250-300 km of lake/river range is required for a self-sustaining population, for example. Wozney et al. (2011) found that habitat fragmentation by dams had not caused any negative genetic impacts in the Ottawa River, presumably because of the long generation time of this species. However, habitats would have to be reconnected for the long-term conservation of the sturgeon.
Age and Growth
Accurate age determination is difficult in long-lived species like sturgeons. Maximum female age is estimated to be 96 years and for males 55 years. There is a report of one fish aged at 154 years from Lake of the Woods. Growth is slower in the north than the south and fish are older. Maturity has been estimated at 8-20 years for males and 14-33 for females, varying with locality. St. Lawrence River female sturgeon reach sexual maturity at an estimated 27 years and 1.33 m and the mean interval between spawnings is 9.4-9.7 years, higher than reports for other populations.
Ottawa River fish mature at 19-20 years and 30 inches (76.2 cm) for males and 26 years and 33 inches (83.8 cm) for females with few males exceeding 45-50 years and females living longer (Harkness and Dymond, 1961). In the Hull-Carillon section of the Ottawa River, 94% of the sturgeon caught in a 1988 study were less than 27 years old (the mean age of first maturity of females) and the species here is not very abundant nor as old, long or heavy as compared to those in the St. Lawrence River (Fournier, 1988; Fortin et al., 1992). Haxton (2002) sampled sturgeons in the Ottawa River over a 5-year period (1997-2001). Three of his areas fall within the NCR - Lac des Chats (above the Chats Falls Dam, mostly upriver of the NCR, sampled 1998), Lac Deschênes (Chats Falls Dam to Chaudière Falls, wholly within the NCR, sampled in 2000) and Lac Dollard des Ormeaux (below the Chaudière Falls to the Carillon Dam, much of it within the NCR, sampled in 2001). Eleven sturgeon were taken in Lac des Chats, none in Lac Deschênes, and 42 in Lac Dollard des Ormeaux. Sturgeon were more abundant in some sections higher up the Ottawa River outside the NCR. The mean total length for Lac des Chats sturgeon was 109.9 cm and mean weight was 8450 g. The mean total length for Lac Dollard des Ormeaux sturgeon was 78.7 cm and mean weight was 2039 g. The latter population showed some signs of recruitment despite being downriver of the NCR. The sex ratio is about 1:1 at birth but by age 40 years it is 6:1 in favour of females. Garvey (2001) found only 8 sturgeon in the disturbed Lac Deschenes over 298.21 hours of fishing compared to 117 fish over 152 hours in the undisturbed Lower Allumette, upriver. Mean total length for Lac Deschenes fish was 121 cm and mean weight was 10.39 kg.
A comparison of the spawning population below the Chats Generating Station was made for sample years 2001-2004 and the study of Dubreuil and Cuerrier (1950) for 1949 fish by Haxton (2006). The spawning stock in 2003 was estimated to be 202 fish, mean size was greater (118.0 cm total length for 2001-2004 compared to 101.7 cm in 1949), weight-length relationships did not vary between studies, and fish less than 110 cm total length comprised only 31.1% of the sample compared to a majority of 69.9% in 1949. This latter observation suggest the population is suffering a recruitment problem. Recruitment did occur because fish were aged at 13 to 46 years, and therefore were not relicts of the pre-1949 population. Ages in the 1949 survey were 15-62 years. Interestingly only males were found in spawning condition, suggesting spawning females could be rare in this section (Lac Deschênes) of the Ottawa River enclosed by dams at Chats and Ottawa.
Haxton (2008) summarises knowledge of sturgeon in the Ottawa River and found the greatest abundance in unimpounded river reaches, a condition found outside the NCR. The von Bertalanffy growth equation for Ottawa River fish was Lt = 133.7(1-exp-0.058(t-(-3))) and condition was described by w = 5.6 x 10-4l3.50. Length and age at 50% maturity was 106.7 cm and 20.4 years fro males and 112.2 cm and 25.4 years for females Fecundity was estimated at 12,170 eggs/kg. Annual mortality was estimated at 15%.
Food
Food is slurped from bottom sediments and includes a wide variety of animal and plant material found in and on the bottom. Sediment is also taken in and ejected from the gill slits or the mouth. The food is detected by the barbels which lightly touch the bottom as the sturgeon swims slowly along. The tubular mouth is protruded as soon as food is detected. Recorded food items are crayfish, other crustaceans, clams, snails, aquatic insects, fish eggs (such as those of Yellow Perch), algae and rarely fish. It is not considered to be a serious predator on the eggs of other fishes. Sturgeon may leap out of the water, a habit attributed to efforts to rid themselves of parasitic lampreys. 61 Silver Lampreys have been recorded on a single Lake Sturgeon, 1.3 m long and 16 kg in weight, caught in the St. Lawrence River, although these were not considered to be enough to kill the sturgeon.
Reproduction
Spawning occurs in April to June at 9-18°C in flowing water or rocky lake margins with wave action. In the Ottawa River at the Fitzroy-Quyon Rapids in 1949 the period was 29 May to 6 June at 56-60°F (10.0-15.6°C) (Harkness and Dymond, 1961). Sturgeon also spawn downstream from Chaudière Falls below Victoria Island in the heart of Ottawa (Haxton and Chubbock, 2002). Peak spawning in Lac des Chats was 12 June in 2006 and 5-15 June in 2007, and in Lac Deschênes was 4-8 June 2001, 4-12 June in 2003 and 8-14 June in 2004. Depths are usually 0.6-4.7 m for spawning generally. Lake populations may migrate up rivers for 400 km to reach spawning grounds although the distance traveled is usually less and fish are generally sedentary outside spawning. Males arrive first on the spawning grounds. The large female is in spawning condition for a short time and is flanked by up to 6 males. The spawning process may involve splashing, vibrations and leaps clear of the water. Eggs and sperm are shed at intervals over a few days and the eggs adhere to rocks. The spawning act lasts only 5 seconds. The eggs are black, up to 3.5 mm in diameter and in very large fish could exceed 3 million. Ottawa River fish produced up to 7179 eggs per pound of fish (Harkness and Dymond, 1961) or 54,000 eggs per kilogramme of ovary (Dubreuil and Cuerrier, 1950). Incubation lasts 5-8 days at 15.6-17.8°C. Spawning occurs at estimated intervals of 1-7 years in males and 4-9 years in females, with longer intervals in the north.
Importance
This species has been used historically for food fresh or smoked, the eggs as caviar, the swimbladder as isinglass (a form of gelatin) and skin as leather. Oil from sturgeon in the Ottawa River has been used by native peoples, mixed with ochre, to delimit pictographs at Oiseau Rock in western Pontiac County, Québec (www.cycloparcppj.org/oiseau/rocheroiseau_a.htm, downloaded 18 April 2005). Haxton (2002) reviews literature that show this species was abundant in the Ottawa River before 1900 and was harvested for food by aboriginal people (see also Gaffield (1997)). Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 63,450 lbs (28,806 kg) in the Ottawa River from Carillon to Pontiac in Québec, the highest recorded. Harkness and Dymond (1961) report past commercial fisheries in the Ottawa River and its lake-like expansions, and the Madawaska River and the Mississippi River and Lake (now absent from the latter two river basins). Commercial fisheries for sturgeons above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b). Fournier (1988) estimated that the fishery in the Hull-Carillon section of the Ottawa River yielded 0.7 kg/ha; Fortin et al. (1992) reporting a catch of about 10 tonnes by three fishermen in this sector. 94% of the catch was less than 27 years old, the age at which more than half the fish are mature. The section of river above Hull contained older fish and exploitation was less strong. The population was overexploited. Ontario Ministry of Natural Resources (OMNR) and Gouvernement du Québec Faune et Parcs (1999) report that a strictly controlled tag and quota system was to be implemented on the Ottawa River to ensure a sustainable harvest rate of 0.1 kg/ha/yr. Ontario fishermen are prohibited from harvesting sturgeon. Séguin (1970) mentions a request to open a commercial fishery on the Gatineau River but this apparently never developed.
Egan (2000) records a commercial harvest of this sturgeon on the Ottawa River as early as 1883 although this is from areas upriver of the NCR near Renfrew and Rolphton. The sturgeon were caught on longlines left overnight. the flesh was smoked, an oil and isinglass extracted, and caviar taken. Gutted sturgeon were sent in dry ice to New York City in the early 1950s and the roe was sent to a packer in North Bay. Today, although commercial fishing for this species is not allowed in the NCR the value of caviar elsewhere is $200/kg and flesh is $40/kg, and by 2006 $400/kg (Butler, 2006). The caviar is reputedly second only to that of Caspian Sea sturgeons.
Dams, pollution and overfishing have reduced populations in Canada. Populations in the Ottawa River were reduced by dam construction which blocks spawning, nursery and feeding migrations, fragments populations and alters habitats, by land clearance for agriculture and logging, by pollution from saw mills and untreated sewage, and by intensive commercial fishing at least in the past (Guénette et al., 1993; Ferguson and Duckworth, 1997; Haxton, 2002; Haxton and Findlay, 2008). Even blasting for a marina on the Ottawa River killed sturgeons around 1970. The Lac Dollards des Ormeaux stretch of the Ottawa River was closed to fishing for sturgeon in 1990 because of a decline in abundance and high contaminant levels (Haxton and Chubbock, 2002). On 1 July 2008 Ontario instituted a zero catch and possession limit on recreational fishing for sturgeon, except for traditional usage.
88,287 kg were caught in 1961 in Canadian waters but the Lake Erie catch alone in the late nineteenth century exceeded 2,268,000 kg. In the Québec portion of the St. Lawrence River commercial yields are very high, up to 3.4 kg/ha with 138.8 tonnes being taken in 1986. These stocks have been over-exploited. They are caught with gill nets, longlines with up to 600 hooks, and seines. One unexpected detrimental factor to sturgeon survival is discarded rubber bands used by Canada Post to bind mail. These are washed through storm sewers into the St. Lawrence River where they become threaded onto the pointed snouts of sturgeon grubbing in the mud for food. Out of 800 fish studied near Québec, 64 had elastic bands. The bands become embedded in the sturgeon's head, interfere with feeding and leave the fish open to infection. Sturgeon with bands weigh one-third less than normal.
It is not a major sport fish in Canada but large specimens are occasionally hooked or snagged to the great surprise of the angler. Some anglers do pursue this giant fish using minnows, meat or worms as bait or spinning gear and hand lines. Sturgeon fight strongly and may leap. There are catches of one sturgeon for every 4 hours fishing effort near Medicine Hat, Alberta. There are winter spear fisheries in the U.S.A. and they are caught through the ice at Petrie Island on the Ottawa River (local informant, 10 February 2007). The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
This species was placed in the "Not at Risk" category in 1986 by the Committee on the Status of Endangered Wildlife in Canada (Houston, 1987) but is listed as threatened in Canada by Peterson et al. (2003).
Lepisosteidae - Gars - Lépisostés
Gars are found in freshwaters of North and Central Americac and Cuba, sometimes entering brackish water and rarely the sea. There are 7 species with 2 in Canada and 1 in the NCR.
Gars have elongate jaws ("gar" is Old English for spear) filled with needle-like teeth. The ganoid scales are heavy, peg and groove hinged, non-overlapping, rhombic and plate-like, forming an effective armour. The tail is abbreviate heterocercal, externally appearing symmetrical but heterocercal internally. An upper tail lobe disappears with growth. There are 3 branchiostegal rays. The swimbladder has a rich blood supply enabling the fish to breathe air through a connection to the gut. A school of gars will break the water surface to breathe air at the same time and reduce the chances of attack by predators. Vertebrae are peculiar in having an opisthocoelous shape - anterior end convex, posterior end concave, a kind of ball and socket joint - which is almost unique in fishes and more usually associated with amphibians and reptiles. Dorsal and anal fins are near the tail. They lack spines but have fulcra (angled scales) on their anterior edge. The alligator gar of the southern U.S.A. and central America is the largest species at 3 m and over 158 kg.
Cretaceous and Eocene fossil gars are known from North America, Europe, India and West Africa. Fossil gars have been reported from Ellesmere Island in the Canadian Arctic Archipelago, well north of their modern distribution. An Upper Cretaceous coprolite (fossil faecal matter) from Alberta contained remains of gar scales and vertebrae. The gar was probably eaten by a crocodile, an indication of the different faunas that Lepisosteus species have lived with in Canada.
Gars favour shallow, weedy areas of lakes and rivers. They are ambush predators, lying still or quietly stalking prey until it can be seized by a sudden rush. Their food is almost entirely fishes. They make excellent subjects for home aquaria when young and for public aquaria when large. Gars are not sought after by anglers since they are hard to hook in their elongate, bony jaws, but a few enthusiasts specialize in their capture. They are not a commercial fishery item. Gar scales and skins are occasionally made into jewelery, picture frames, purses and boxes. The scales can be highly polished.
Longnose Gar / Lépisosté osseux
Lepisosteus osseus (Linnaeus, 1758)
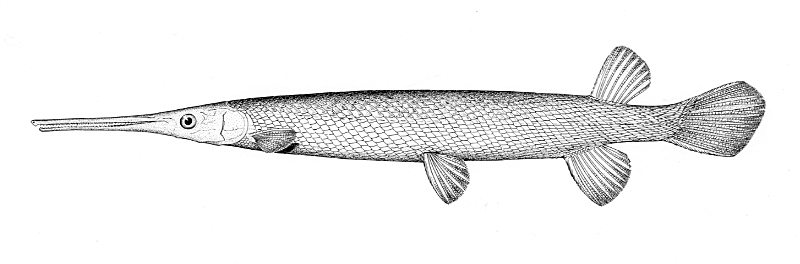
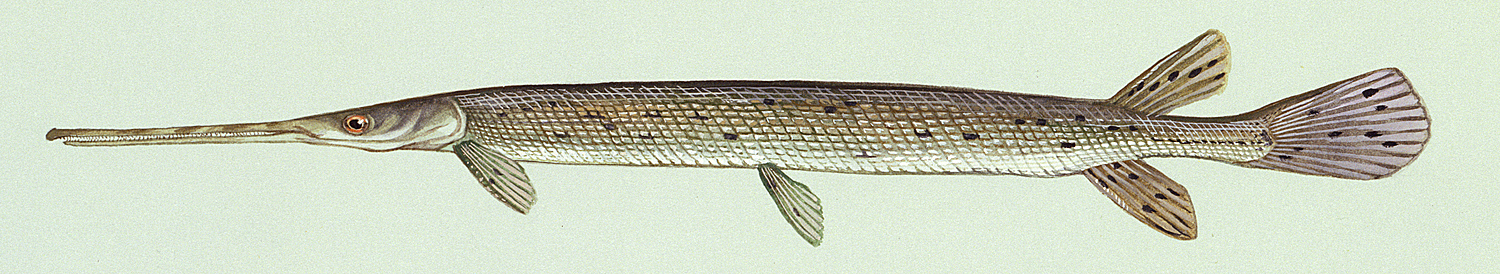



Taxonomy
Other common names include Garpike, Northern Longnose Gar, Billy Gar, Billfish, Needlenose, Northern Mailed Fish, Pin-nose Gar, Bonypike, Scissorbill and Poisson armé.
Key Characters
This species is the only gar in the NCR and is readily identified by the very elongate snout armed with needle-like teeth.
Description
This species is distinguished by the long, narrow snout 14-18 times longer than minimum width, 57-66 lateral line scales and spots only on the body from the pelvic fins to the caudal peduncle and on the dorsal, anal and caudal fins. Gill rakers 14-31. Dorsal fin rays 6-9, anal rays 7-10, pectoral rays 10-13 and 6 pelvic rays. Young fish have dorsal and ventral filaments on the caudal fin. The swimming young fish appears to be moved by a propeller as these filaments vibrate rapidly.
Colour
Adults are grey or olive-brown to dark green fading to pale green or silver on the flanks and white on the belly. Colour is variable with habitat. Flank scales often outlined in black. The dorsal, anal and caudal fins are pale brown to yellow and spotted. Pectoral and pelvic fins are dusky without spots. Young have a narrow reddish-brown or black stripe on the back, and one on the mid-flank which has a wavy upper edge. Above the flank band they are brown to black, below brown with white or cream areas.
Size
Reaches over 2 m and 37.2 kg. The world, all-tackle angling record weighed 22.82 kg and came from the Trinity River, Texas in 1954, but this may have been another species, probably an Alligator Gar. A gar weighing 16 lbs (7.3 kg) and measuring 40 inches (1.02 m) in length was caught near Rideau Falls in the Ottawa River (Anonymous, 1974b), a 13.9 lbs (6.3 kg) gar from the Ottawa River on 3 July 1973 ( www.canadian-sportfishing.com/NationalFishRegistry/Catch_And_Keep1.asp), a 15.2 lbs (6.9 kg) fish was caught by Scott Thibeault in the Ottawa River on 1 June 2001 (www.ofah.org/Registry/fish.cfm?RecID=23, downloaded 14 May 2004), a 51 inch fish with a 16 inch girth was caught by Jeff Cyr in the Ottawa River on 6 October 1994 ( www.canadian-sportfishing.com/NationalFishRegistry/Live_Release1.asp, downloaded 13 June 2003), and gar about 20 lbs and 54-57 inches long are reported form the Ottawa River (G. Barnardo, in email, 10 June 2004).
In Canada found from the St. Lawrence River basin, the Great Lakes, but rare in Lake Superior, across southern Ontario. In the U.S.A. found in the Mississippi River, Great Lakes and southern Atlantic coastal basins, absent from the eastern American mountains. In the NCR it is found in the Ottawa River and mouths of tributary rivers.
Origin
This species entered the NCR from a Mississippian or possibly an Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
It is usually found in quiet, weedy shallows of lakes and larger rivers and, because of its air-breathing ability, enters hot stagnant waters to feed where other predators could not survive. The preferred temperature is said to be 33.1°C. Gars can be seen in summer hovering motionless at the surface although they dive rapidly out of sight if disturbed. They can also be seen at night in summer when canoeing in Shirley's Bay, the most common mid- to large-sized fish in the bay (Eric Snyder, pers comm., 7 January 2008).
Age and Growth
Maximum age is 27 years for males and 32 years for females. Females grow faster and the sex ratio of males to females changes from about 262 to 100 in early life to only 8 males per 100 females after age 10.The growth of young is the fastest of any North American freshwater fish. These gar are mature at 6 years and 50.0 cm.
Food
Food is usually all fishes of suitable size, more rarely frogs, crayfish and even small aquatic mammals. Prey is seized by a sideways slash of the snout after a dart or drift from cover. The prey, impaled on the teeth, is manoeuvred so that is can be swallowed head first.
Reproduction
Spawning occurs in spring and summer at 20°C or warmer usually in weedy shallows. Ripe gar that were presumed to be spawning were caught in the lower Carp River, Fitzroy Harbour Provincial Park on 10 June 1964 (McAllister and Coad, 1975). Water temperature was 23°C and the current medium over a rocky bottom. They were still spawning here in 2009, on the afternoon of June 6th (the night of a full moon), and in fewer numbers on the following morning. Stewart Fast (pers. comm., 19 June 2009) observed spawning of up to 200 gar swimming on top of each other, at times 4 or 5 deep, in 75 cm deep pools downstream of a small waterfall. Orange to brown eggs were seen on rocks and plants. Campers were able to pick up fish by hand. Some fish were an estimated 125 cm long. Gar are also reported as spawning in a large pool in the Ottawa River at the end of Woodroffe Avenue in June (www.ottawariverkeeper.ca/riverkeeper/ecology/@200_river/phen.html) and on 13 June 2008 in the Quyon River at the lower bridge (see below). The female is approached by up to 15 males which she leads in an elliptical path. The males nudge the female's belly area with their snouts while oriented head down. Males and female quiver and eggs and sperm are released. The eggs are scattered and attach to vegetation. The eggs are dark green, perhaps as camouflage, and measure 3.2 mm in diameter. The number of eggs may be as high as 77,156. Eggs are poisonous to birds, mammals and humans, and can kill smaller mammals. In Ontario gar eggs have been found in the nests of Smallmouth Bass and such nests had a higher success rate than nests with only bass eggs. Whether gar are deliberately spawning over bass nests is uncertain, they may merely be in the same area. However they do gain an advantage because the bass defends the nest against predators. Curiously, gar eggs are eaten by other fish despite being reputedly poisonous to mammals. The bass may benefit by having larger gar eggs and larvae in the nest to distract predators from the smaller bass eggs and larvae. Also more eggs and larvae of whatever species lessens the chance of individual loss to a single predatory attempt. Conversely, the male bass has more eggs to guard and the larger gar eggs are more attractive to predators, but on balance both fish species benefit. The young gar have an adhesive pad on the snout tip which attaches them to weeds. After about 9 weeks the yolk-sac is absorbed, the gar no longer hangs vertically from vegetation and is free-swimming. They grow very rapidly, as much as 3.9 mm a day, much faster than most other Canadian freshwater fishes.


Importance
Gar are considered a pest because they eat other fishes and are often killed by fish. They have been pursued in the NCR by anglers who specialise in trying to catch this species with its narrow bony mouth. They are edible, better tasting when smoked, although they are hard to clean because of the bony armour. The flesh must be carefully cleaned of the eggs before eating as these are thought to be poisonous although there is some dispute about this.
Amiidae - Bowfins - Poissons-castors
The Bowfin is found in freshwaters of eastern North America and is the only member of its family. Fossil amiids are known world-wide and the oldest are of Jurassic age, 135-195 million years ago. Eocene amiids have been described from British Columbia, Palaeocene and Cretaceous ones from Alberta and Palaeocene and Oligocene ones from Saskatchewan. The genus Amia has been extant for 70 million years; evolutionary change is very slow.
The general body form of a Bowfin is unmistakable. In addition there is a large bony structure on the underside of the head between the lower jaws known as a gular plate. Branchiostegal rays number 10-13. There are no pyloric caeca. The caudal fin is an abbreviate heterocercal one. Heterocercal tails have the vertebral column turned upwards into the upper lobe of the fin, which is longer than the lower lobe. In the Bowfin, the lobes are not noticeably different in size. Scales are cycloid but are reinforced with ganoin. Some teeth are pointed canines while others are peg-like. Gill rakers are reduced to knobs but bear small spines.
The Bowfin's relationships to other fishes have long been discussed and large monographs have been written on the details of its anatomy. It is, in a sense, a living fossil, since many related families and species were widespread in the Jurassic and Cretaceous periods, but the Bowfin is the only surviving representative. Unlike sturgeons, the skeleton is bony but it has the heterocercal tail and a trace of a spiral valve. The gular plate, heavy bone plates on the head, and ganoin containing scales are also ancient characters. It is now considered to be related to the Teleostei and, with its fossil relatives, is equal in rank to the thousands of teleost species. The Bowfin swimbladder can be used as a lung since it has an opening to the gut and the internal surface is well-supplied with blood vessels. This fish can survive out of water for a day, and thrives in low oxygen waters such as stagnant swamps. Recent studies have shown that Bowfins cannot aestivate like the tropical lungfishes because they cannot detoxify ammonia waste or reduce metabolism and they die after 3-5 days of air exposure.
Bowfin / Poisson-castor
Amia calva Linnaeus, 1766
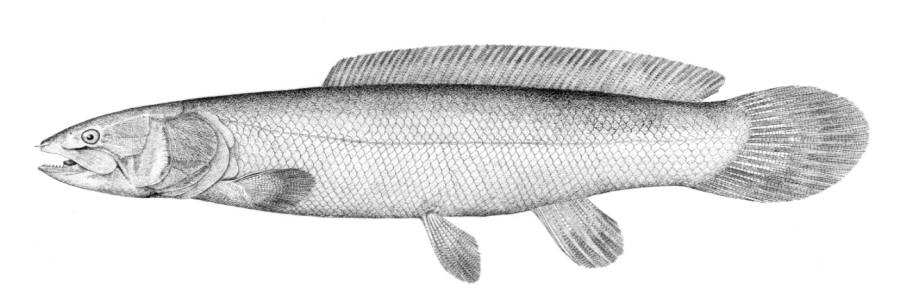


Taxonomy
Other common names include Dogfish, Mudfish, Mud Pike, Grindle, Grinnell, Griddle, Spot-tail, Lawyer, Cottonfish, Blackfish, Speckled Cat, Scaled Ling, Beaverfish, Cypress Trout, Amie, Poisson de marais and Choupique. Bowfin refers to the long, undulating dorsal fin.
Key Characters
The gular plate, a large bony structure between the lower jaws on the underside of the head, identifies this freshwater fish.
Description
The dorsal fin has 42-58 soft rays, the anal fin 9-12 rays and the pectoral fin 16-18 rays. There are 62-70 scales in a complete lateral line. The anterior nostrils have barbel-like flap.
Colour
The back is a dark-olive or brownish with the flanks mottled, marbled or reticulated with olive and yellow. The belly varies from white to light green. The dorsal fin is dark olive with 2 dark broken stripes, the anal, pelvic and pectoral fins are bright green. Males have an eye spot at the upper caudal fin base. The spot is dark and about twice as large as the eye and is surrounded by an orange or yellow halo. This spot is absent in females. Such eyespots are used to deflect the attack of predators from the eye to the less important tail, which may well give the predator a slap in the face! The anal, pectoral and pelvic fins have orange bases and tips in males. Young fish are lighter overall and have a black margin to the dorsal and caudal fins. There is a narrow stripe from the snout through the eye onto the opercle. Young smaller than 3-4 cm are black.
Size
Attains 109 cm and 9.87 kg. The world, all-tackle record from Florence, South Carolina in 1980 weighed 9.75 kg and a 6.7 kg fish was taken from Whitefish Lake, Ontario.
Found from the St. Lawrence and Lake Champlain drainage of southern Québec westward around the Great Lakes in southern Ontario as far as Minnesota. In the south it reaches Florida and Texas. There are no specimens in a museum collection definitively from the NCR. Halkett (1906) mentioned two specimens from the Ottawa River in the Fisheries Museum, Ottawa and Prince et al. (1906) also reported two specimens in the Museum from the Ottawa River (presumably the same two fish) but did note they may not have been caught near "the district". Bergeron and Brousseau (1982) and Bernatchez and Giroux (2000) map this species within the NCR but may have based this on Prince et al. (1906). However competent fishermen familiar with a wide variety of fishes have reported it from Britannia in the late 1940s, Rockland; and even below the Parliament Buildings, all in the Ottawa River (J. McLoughlin, personal communication, 1986; D. Brunton, in letter, 1987; E. Hendrycks, personal communication, 2000). Chabot and Caron (1996) map a specimen from the Ottawa River, near the mouth of the Gatineau River.
Origin
This species entered the NCR from a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
Bowfins prefer warm quieter waters with a lot of vegetation in lakes and river backwaters. They can survive temperatures up to 35°C in stagnant waters which other predatory fish cannot utilise. However they can be found too in clear water bodies that are quite cool. Bowfins gulp air at the surface even in well-oxygenated water. Their preferred temperature is 30.5°C. Bowfins can aestivate for short periods in a moist chamber, 20 cm in diameter and 10 cm below the soil surface when flood waters recede.
Age and Growth
Life span may exceed 30 years. Males are smaller than females and probably do not live as long. Bowfins become sexually mature at 3-5 years of age when they are about 61 cm (females) and 45.7 cm (males). Growth is rapid with some fish exceeding 20 cm in the first year of life.
Food
Food is mainly other fishes, with some crayfishes, aquatic insects and frogs, taken at night after moving into shallower water. The Bowfin feeds by a rapid lunge, opening the mouth to suck in the prey. The opening and closing of the mouth takes about 0.075 seconds. They can also move very stealthily by undulating the dorsal fin, moving both backwards and forwards.
Reproduction
This species spawns from April to June depending on latitude. Nests are constructed by the male in shallow (usually less than 1.5 m), weedy areas of lakes and rivers. The nests are under logs or other objects, or are circular areas up to 76 cm across where all vegetation has been bitten off and removed. The male defends his nest against other males and during the spawning season torn fins are not unusual as nests may be quite close together. Spawning takes place at 16-19°C when the male entices a female into the nest, circles her for 10-15 minutes while she lies on the bottom of the nest, and nips her snout and flanks. The male then lies with the female, their fins vibrate rapidly and eggs and sperm are released within a minute. This may happen 4-5 times over 1-2 hours. Several females may spawn with one male and each female may deposit eggs in more than one nest. Eggs number up to 64,000 in females but number up to 5000 in nests. They stick to the plant roots or gravel in the bottom of the nest. The male guards the eggs and fans them with his pectoral fins. Eggs are 2.8 x 2.2 mm in dimensions. The eggs hatch in 8-10 days and the young use an adhesive snout organ to attach to vegetation for a further 7-9 days while the yolk-sac is absorbed. The male continues to guard and herd the young fry until they are about 10 cm long. The fry form into a ball which follows the male. So defensive are males, that one attempted to attack a human standing on the bank, coming 20 cm or so out of the water and repeating the attack several times.
Importance
The Bowfin is a good sport fish on light tackle, but is seldom fished for and is very rare in the NCR. Some are taken elsewhere by spearing while diving. It is edible, though not particularly tasty, and some commercial catches in Ontario have been sent to the United States where it is a more familiar food fish and better appreciated. "Cajun caviar" is made out of the roe in Louisiana. Small Bowfins are excellent aquarium fish because of their "lung", colouration and predatory habits.
Hiodontidae - Mooneyes - Laquaiches
The Mooneyes are found only in North American fresh waters in central Atlantic, Arctic and Gulf of Mexico drainages. There are only 2 species, both found in Canada, with 1 in the NCR.
These moderate-sized fishes are similar in appearance to Herrings but have teeth on the tongue, roof of the mouth and jaws, and a dorsal fin far back over the elongate anal fin. There are 7 pelvic fin rays, a pelvic axillary process, 7-10 branchiostegal rays, a subopercular bone is present on the side of the head and scales in the lateral line number 51-62. There is a ventral keel to the body but no scutes as in herrings. Scales are cycloid. The swimbladder is connected to the skull. The eyes are large and far forward on the head near the rounded snout. There are adipose eyelids. There is a single pyloric caecum.
A unique feature is that eggs are ovulated directly into the body cavity and not carried externally via oviducts as in most bony fishes.
Mooneye / Laquaiche argentée
Hiodon tergisus Le Sueur, 1818
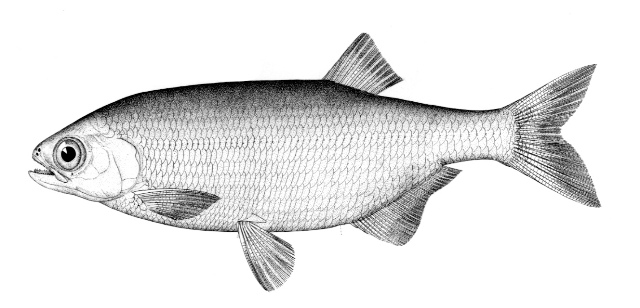
Taxonomy
Other common names include Toothed Herring, River Whitefish, Freshwater Herring, Cisco and White Shad. Locally called whitefish (Hopkins, 2000).
Key Characters
This species is the only member of its family in the NCR and is characterised by having a fleshy ventral keel from the pelvic to the anal fins, a dorsal fin origin in front of the anal fin origin, a long anal fin, a pelvic axillary process, no adipose fin, teeth in the jaws, and the mouth extending at most to mid-pupil level.
Description
Dorsal fin rays 10-14, anal rays 26-33 and pectoral rays 13-15. Scales in lateral line 51-60. Gill rakers 11-17. The eye is large. The anal fin base has 2-3 rows of small scales and, in males, the anterior part of the anal fin is greatly enlarged leaving the margin behind strongly concave. Males also show a concavity on the body over the anterior anal fin base rather like a depression caused by a pressed thumb.Colour
Colour is olive to brown on the back with a steel-blue sheen, flanks are silvery and the belly white. Fins are dusky, and a black stripe margins the leading edge of the pectoral fin. Spawning males and females have a pinkish hue on their fins and bellies. The eyes are golden above and silvery below.
Size
Reaches 47.0 cm and 1.1 kg. A Bay of Quinte, Ontario fish weighed 0.65 kg and is the Canadian fishing record.
Found from the James Bay lowlands of northeastern Ontario and adjacent Québec, south through the Ottawa River basin to the upper St. Lawrence and Lake Champlain, and lakes Ontario and Erie. Also in Lake of the Woods, southern Manitoba and Saskatchewan and the North and South Saskatchewan, Battle and Red Deer rivers of Alberta. In central U.S.A. south to the Gulf coast. In the NCR long known only from the Ottawa River but now recorded from the South Nation River at Casselman (29 May 2004).
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Mooneyes are found in both running and still shallow waters of lakes and rivers and appear to be sensitive to turbidity. They are usually taken at less than 11 m depth. Their preferred temperatures are 22-27°C. There is a migration up rivers to spawn in the spring.
Age and Growth
Males mature as early as 3 years while females are mature at 4-5 years, generally. Talajic (1980) studied this species in the Ottawa River above and below Ottawa. Life span is up to 13 years in the Ottawa River using scales for ageing verified by reading otoliths and vertebrae. Females live longer than males, the latter reaching 11 years in the Ottawa. Females mature at age 4 above and below Ottawa while males mature at 3 years (below Ottawa) and 4 years (above Ottawa). Females outnumber males by 2.2:1 (below Ottawa) and 3.4:1 (above Ottawa). Males and females showed no significant differences in length, weight and age although fish below Ottawa were significantly smaller than fish above Ottawa at an age. Growth is similar to other mooneye populations in Canada.
Food
Food includes insects, crustaceans such as crayfish and plankton, molluscs and small fishes. Ottawa River Mooneyes fed on ants, mayflies, dragonflies and beetles based on stomach contents (McAllister and Coad, 1975). Talajic (1980) found that the most important foods in the Ottawa River were Ephemeroptera, Trichoptera, Diptera and Coleoptera with smaller amounts of other aquatic insect larvae and, rarely, minnows, clams, spiders and crustaceans. Flying ants were also found in large numbers. Food depended on availability, varying through the year. Feeding often occurs at the surface in the evening and during the night when insects fallen on the water surface are taken aided by the light-sensitive eyes.
Reproduction
Spawning takes place in April-June and each female may produce up to 20,000 buoyant, blue-grey eggs of 2.1 mm diameter deposited over rocks and gravel in running water. Ripe females have been caught at the beginning of June near the mouth of the Gatineau River (McAllister and Coad, 1975) and Talajic (1974) found spawning from mid-May to mid-June at 10-14ºC. Talajic (1974) recorded up to an average of 9000 eggs of average diameter 2.24 mm, with the largest eggs to 2.98 mm. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 10 Mayl to 23 June at 12.5-19ºC.
Importance
This species is of limited commercial importance, mostly on the U.S. side of Lake Erie. Various Herrings and Whitefishes have been listed erroneously as Mooneye in catch statistics. Commercial fisheries for mooneyes above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b), assuming "Laquaiche aux yeux d'or" is a mis-nomer for this species. Ontario Ministry of Natural Resources (OMNR) and Gouvernement du Québec Faune et Parcs (1999) report one license is issued for this species in the Ottawa River (Carillon to Ottawa-Hull). It is caught by anglers who specialise in catching this unusual sport species using flies, worms, grasshoppers, minnows or lures on light tackle. It is best eaten spiced, smoked or fried with butter and onions as it is dry and tasteless when fresh
Anguillidae - Freshwater Eels - Anguilles d'eau douce
Freshwater eels are found world-wide in temperate to tropical waters except for the south Atlantic Ocean and the whole eastern Pacific Ocean. There are 16 species with 1 occurring in Canada and the NCR.
The term eel-like is based on the body shape of freshwater eels and includes the muscular slipperiness associated with this fish and its mucus-producing skin. Both dorsal and anal fins are long and join the tail fin. The dorsal fin begins well behind the pectoral fin level. There are no pelvic fins and the pectorals, when present, are on mid-flank. Scales are absent or when present small, embedded and cycloid. There is a lateral line. Jaws are strong and toothed. The gill openings are small and just in front of the pectoral fins. The anterior nostril is tubular
The life cycle of eels was unknown until Johannes Schmidt published his 1922 study based on years of collecting. Where the adults went on their seaward migration and where the elvers ascending rivers came from were a mystery. These eels are catadromous, living in freshwater but migrating to the sea to spawn and die. In the North Atlantic Ocean spawning occurs in the Sargasso Sea. The young eels or leptocephali (= thin head larvae) are distinctive being transparent and leaf-like. A newspaper can be read through the body of a leptocephalus. In this form they drift to the shores of America and Europe, transform into elvers with the more familiar eel-shape and move into rivers and lakes to feed and grow.
The biology of eels is based almost entirely on the freshwater phase of their life. Adults in freshwater develop large eyes, the gut degenerates and coloration changes in preparation for the migration to the Sargasso Sea. Adults were only caught in the deep ocean, at nearly 2000 m near the Bahamas, in 1977. The Sargasso spawning ground is deduced from collections of larvae across the Atlantic Ocean - the smallest and youngest larvae are found around the Sargasso Sea. The spawning grounds are at about 400 m, at a 17°C temperature and in saltier water than usual sea conditions according to some authors but since spawning adults have never been caught this remains dubious.
The theory advanced by D. W. Tucker in 1959 maintained that European Eels lack the energy resources in their migratory, spawning phase to reach the Sargasso Sea 7000 km from Europe. They are presumed to be following an instinct to head out to sea, dating from an earlier geological age when the Atlantic Ocean was narrower before the separation caused by Continental Drift. All European Eels die at sea and Europe is restocked by larvae drifting there spawned from American parents. The American populations are closer to the Sargasso and can make the journey easily. Differences between American and European eels are merely the consequence of different environmental regimes in different parts of the Sargasso. This theory has not found general acceptance but, if true, means that all European Eels can be harvested for food without depleting stocks. Eels are valued as food, particularly in Europe and Japan, but are not used as extensively in North America.
American Eel / Anguille d'Amérique
Anguilla rostrata (Lesueur, 1817)
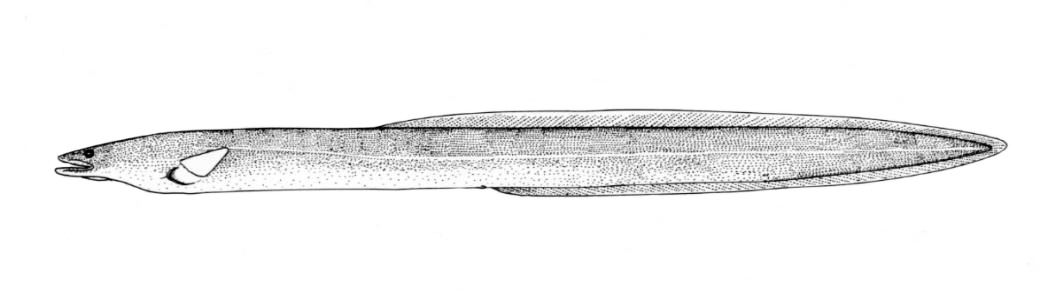

Taxonomy
Other common names include Common Eel, Atlantic Eel, Boston Eel, Snakefish, Silver Eel, Yellow-bellied Eel, Bronze Eel, Black Eel or Green Eel and Anguille argentée.
Key Characters
The American Eel is distinguished by its shape, confluent dorsal, caudal and anal fins, the toothed jaws, and the single gill opening. Lampreys have a similar shape and confluent fins but have no jaws (teeth are in a sucking disc) and there are 7 gill openings.
Description
Scales are small and embedded in the skin and not readily visible without close examination. The dorsal fin has about 240 rays, the anal fin somewhat fewer around 200, and the pectoral fin has 14-20 rays. Branchiostegal rays number 8-14 and vertebrae 103-112. The lower jaw projects and the mouth extends to the rear, or beyond, of the eye. The gill opening is a small slit in front of the pectoral fin. Migrating fish change colour and the eyes of males almost triple in size.
Colour
Freshwater adults are overall yellow, greenish, muddy or olive-brown with a dark back, sometimes yellow, green, orange or pink flank tinges and a creamy or yellowish-white belly. Such adults are called Yellow Eels. Adults migrating to the sea have a bronze to black back, a metallic sheen and a light to silvery belly. They are then known as Silver, Bronze or Black Eels. Colour will also change gradually to match the substrate. The larvae are transparent, the transformed elvers or glass eels are also transparent (with black eyes) but soon become grey-green to black, but only adults are found in the NCR.
Size
Reaches 1.52 m and 7.5 kg in females and 50.3 cm in males. Fish larger than 40 cm total length are almost always females. The world, all-tackle angling record weighed 3.88 kg and was caught at Cliff Pond, Massachusetts in 1992. A 5.1 lbs (2.32 kg), 38.5 inch (97.8 cm) long and 9.25 inch (23.5 cm) girth was caught in the Ottawa River on 30 July 2002 by Kyle Richards (www.ofah.org/Registry/fish.cfm?RecID=1, downloaded 14 May 2004).
Found in the western North Atlantic from central Labrador south to Brazil. Also in freshwater drainages of the Mississippi and Great Lakes basins, the Hudson Bay drainage of Alberta and Saskatchewan (by introduction) and throughout Maritime Canada. Eels are reported from the Mississippi Lake and therefore can be expected to occur in the Mississippi River of the NCR as well as the Ottawa River. Eels were once so numerous at the Chaudiėre Falls in Ottawa that they stopped a turbine mill wheel - numbers of eels were estimated in the hundreds of thousands (Kerr, 2010a). Eels have been caught in the Plantagenet and Berwick reaches of the South Nation River (not on map; South Nation Conservation Authority (2010)).
Origin
This species entered the NCR from an Atlantic coastal refugium.
Habitat
Eels are found in mud-bottomed rivers, streams and lakes. They can be seen looped over weeds in rivers but are usually nocturnal and lie buried in mud during the day. Their preferred temperature is 19.0°C. In winter they bury themselves in mud and are torpid. They can travel overland to reach isolated water bodies, using snake-like movements when the ground is wet. Elvers can climb short vertical, wet walls such as those at canal locks. The Moses-Saunders Hydroelectric Power Dam on the St. Lawrence River at Cornwall was a barrier to young eels migrating into Lake Ontario. An eel ladder (a trough and baffle system with rest pools crisscrossing an ice chute through the dam) was built and over 3 million eels used it in 4 years. There is some evidence of a homing ability to their river of origin if they are displaced. In Lake Ainslie, Nova Scotia, eels have been observed in clumps or eel balls of up to 30 fish found on the bottom or even breaking the surface. Eels can be heard making chirping or sucking noises in warm summer weather on Cape Breton Island. Silver Eels in Newfoundland migrate to sea at age 9-18 years. They leave Nova Scotian waters in late August to mid-November. Adults in Passamaquoddy Bay, N.B. are active by day and night in contrast to freshwater eels, and made frequent surface to bottom dives perhaps to sample geoelectric fields as orientation cues for migration. Migration is often nocturnal.
Age and Growth
Life span is at least 43 years, possibly 50 years or more. Males mature at about 28-30 cm and females at 46 cm. Females grow larger than males, as much as twice the length. The leptocephalus larva has a 1 year life span in the sea.
Food
In freshwater food is any bottom invertebrates, frogs and fishes. Smaller eels favour insects but large eels eat fish and crayfish as well as carrion. In New Brunswick eels are important predators of Atlantic Salmon in nets or traps. Elvers are cannibals. A wide variety of fishes and birds eat eels at various life stages. When they migrate back to the sea, they stop feeding.
Reproduction
Spawning in the Sargasso Sea is believed to occur from February to July but has not been observed. Egg numbers have been estimated as up to 20 million per female but the number released is guesswork as none have been found. The adults take 2-3 months to reach the Sargasso and the adults die after spawning. The Sargasso spawning grounds of the American Eel are to the southwest of the European Eel grounds although there is evidence for a more southern spawning ground. Leptocephali take 1 (perhaps more) year to drift to Canadian shores and transformation to a young eel or elver occurs at 60-65 mm during winter while drifting to or in nearshore waters. Glass eels near Saint John's, Newfoundland have been observed about 2 m below the surface drifting heads up and tails down. This may be done as camouflage from predators which swim horizontally, to counteract sinking, to escape vertically from predators and to facilitate vertical migration. The elvers enter estuaries in April and are 65-90 mm long. They are found in coastal rivers from May to July. The run lasts from a few days to several weeks depending on the river. In the northern Gulf of St. Lawrence young eels only move upstream in their second summer of stream residence. Temperature may be a factor in successful elver arrival. Males tend to stay near the coast in estuaries while females move up rivers sometimes as much as thousands of kilometres. All 356 eels sexed in Lake Champlain were female for example. Males also appear to be much rarer than females in northern waters. Males may have this distribution to ensure rapid maturation and return to the spawning ground. They do not need to be large to produce adequate amounts of sperm and estuaries are good feeding areas. Females require a delayed maturation as a larger body size results in more eggs. Cold northern and inland waters favour this. There are different stocks of eels in Canadian waters. Lake Ontario eels can be distinguished from those in St. Lawrence River tributaries and the Maritimes by the presence of mirex, a chemical used in insecticides. Pollutants are now a convenient method for stock identification but a sad reflection on the state of the environment.
Importance
Eels can be a nuisance to fishermen as they eat other fish caught in nets. They are used in physiological and other studies in laboratories and are easy to keep, surviving without food for up to 22 months. Adult eels are caught in Canada for export to Europe, particularly along the Gulf of St. Lawrence, using weirs, baited setlines, and pots, fyke nets, eel traps and hoop nets. Some are speared during winter when they are buried in mud. The catch in Lake Ontario reached 221,940 kg in 1978 and the total Quebec fishery for the same year was 527.9 tonnes and for the Maritimes about 320 tonnes. The total Canadian catch in 1988 was 1016 tonnes, worth about $2.3 million. Increased fishing pressure in Lake Ontario resulted in a decrease in average eel size. Elvers have been caught in Canada for raising in ponds in the U.S.A. and the Far East. Efforts to start such aquaculture in Canada have not met with extensive success but are potentially viable. Eels are exported live, on ice, or frozen. Live eels are used in making jellied eels which are popular in England. Smoked eel is an important, tasty and highly priced product. Eels should be cleaned with care as the blood has a neurotoxin which can affect humans but is destroyed in cooking. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi River and Ottawa River. As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 8172 kg in the Ottawa River from Carillon to Pontiac in Québec, the catch in 1933 from Hull, Labelle and Pontiac counties was 16,162 kg, and the catch in 1921 in Prescott, Russell, Carleton and Renfrew counties was 1800 kg, all the highest figures recorded. Generally catches were greater on the Québec side because there were more commercial fishermen there than on the Ontario side. Commercial fisheries for eels above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b). Lac Dollard des Ormeaux, the stretch of the Ottawa River form the Chaudière Falls to the Carillon Dam, had a harvest of 295 kg by Ontario commercial fishermen in 1999 but only 69 kg in 2000 (Haxton and Chubbock, 2002).
Blasting for a marina on the Ottawa River killed large eels around 1970.
Eels are considered to be critically imperilled in Ontario and since the South Nation River is the first watershed of the Ottawa River above the Carillon Dam, it may prove important for future stewardship of this species (South Nation Conservation Authority, 2010).
Clupeidae - Herrings - Harengs
Herrings, shads, sardines, pilchards and menhadens are found world-wide in warmer marine waters with some species anadromous or permanent freshwater residents. There are about 180 species with 9 found in Canada, but only 1 occurs in the NCR.
These fishes have modified scales on the belly forming abdominal scutes with a saw-like edge. The lateral line is usually absent or on only a few scales. Silvery cycloid scales are easily detached and are found only on the body. Teeth are small or absent but gill rakers are long and numerous for sieving plankton. Fins lack spines. There is no adipose fin. The pectoral and pelvic fins have a large axillary scale. The caudal fin is deeply forked. The eye is partly covered by an adipose eyelid. The flesh is particularly oily and is highly nutritional.
Herring are easily caught and are extremely valuable to commercial fisheries. They are the most important fishes economically, both as food for man and also for many other commercial fish species. An estimated 10 billion Atlantic Herring are caught each year and in one year members of the herring family made up 37.3% of all fish caught in the world. Some are used for fish meal, as fertiliser and as an oil source.
Dymond (1939) mentions the American Shad (Alose Savoureuse, Alosa sapidissima (Wilson, 1811)) as occurring in the "Ottawa Region" but this probably refers to the lower Ottawa River outside the NCR. The construction of the dam at Carillon has blocked further upstream migration in recent years and the rapids there before the dam was constructed probably limited access to a few strays, perhaps none of which reached the NCR.
Alewife / Gaspareau
Alosa pseudoharengus (Wilson, 1811)


Taxonomy
Other common names include Gaspereau, Mulhaden, Sawbelly, Spreau, Kyack, Grey Herring, Glut Herring, Branch Herring, Spring Herring, Golden Shad and River Herring.
Key Characters
This herring is the only member of the family in the NCR. It is distinguished by the row of sharp-edged scales on the belly, the adipose eyelid, no lateral line or adipose fin, and scales extending onto each lobe of the caudal fin.
Description
Dorsal fin rays 12-19, anal rays 15-21, pectoral rays 12-16 and pelvic rays 8. Scales along flank 42-54. Gill rakers long, numbering 38-46. Belly scales before the pelvic fin 17-21, behind 12-17.The dorsal fin origin is not far forward of the pelvic fins, there are no enlarged scales before the dorsal fin on the back, there are no teeth on the roof of the mouth, the lower jaw projects and does not fit into the upper jaw notch, eye diameter is longer than the snout length, and there is no diamond-shaped scale pattern, as in related species.
Colour
The back is grey-green and the sides and belly are iridescent silver. The flank may have copper tinges in sea-run specimens or violet tinges in young. Lines run along the flank above the mid-line in some adults. There is a black spot behind the head on the upper flank. Fins are pale yellow or green but can be darkish. The caudal fin is dark with a white leading edge to the lower lobe. The peritoneum is silvery, pearl or pinkish-grey with small spots.Size
Attains 40.0 cm and 0.28 kg.
Found from the Gulf of St. Lawrence and northeastern Newfoundland to South Carolina in the sea and tributary rivers. Also in the Great Lakes and tributaries having entered the higher lakes via canal systems. Known only from 2 collections from the Rideau Canal below the locks at Hog's Back Road, Ottawa (Coad, 1983b).
Origin
This species entered the NCR from the Rideau River system where they are known northeast of Kingston. None have been reported from the Ottawa River above the Carillon Dam (Coad, 1983b).
Habitat
The Alewife is a marine species which enters freshwater to spawn but some freshwater populations are land-locked and no longer return to the sea. They are found in both rivers and lakes where their preferred temperature is 18.8°C. The spread of Alewife through the Great Lakes via canals has been rapid. The first appearance in Lake Erie was in 1931, in Lake Huron 1933, and it is now abundant in both these lakes.
Age and Growth
Alewife mature at ages 3-5 with males maturing earlier than females, sometimes as young as 1-2 years. Females are longer and heavier than males of the same age. Life span exceeds 10 years.
Food
Food is the larger zooplankton, items of which may be selected individually, by a dart and suck, as well as filtered indiscriminately while swimming along with the mouth open. Alewife may choose either method depending on available food size. They may also gulp concentrations of plankton. In freshwater zooplankton is eaten too but some bottom amphipods are also taken and the diet may include aquatic insects in late summer when zooplankton numbers fall off. Larval fishes are part of the zooplankton and in freshwaters Alewife may be an important predator on commercial and sport fishes. Introduced Coho Salmon are an important predator on Alewife in the Great Lakes and have reduced mass die-offs.
Reproduction
Spawning occurs in freshwater in spring and early summer (April-July) at water temperatures as low as 8.9°C. The pre-spawning migration in the St. John River, N.B. starts 3 months before spawning. Slow rivers are favoured but spawning does occur in lakes, ponds and streams. In lakes there is a spawning migration from deep water onto beaches in April-July, the timing being dependent on temperature. Spawning occurs in Lake Ontario at 13-16°C. Two or more fish swim in tight circles with flanks touching, rising to the surface. After circling once or twice at the surface, the fish dive and presumably release eggs and sperm over the substrate. Spawning takes place in the evening and at night. Older fish spawn first and may be spawning for the fifth time. Eggs are 1.3 mm in diameter and each female may produce 450,000 eggs. Young return to the sea in late summer and fall. The downstream migration is triggered by increasing rainfall, rapid decrease in water temperature (below 12°C) and the moon phase (dark nights).
Importance
Alewife are commercially important and are caught on the spawning run using weirs, gill nets, dip nets and traps. The 1980 catch, which included Blueback Herring, was 9738 tonnes valued at $2,482,000. The 1988 catch of Alewife alone was 5560 tonnes. They are sold fresh, frozen, smoked, salted or pickled. Some is used as pet food, fish meal or bait in angling and for lobster and snow crab. The flesh is good but bony. Landlocked specimens are thinner and smaller and so are not used much as food for people. In the Great Lakes mass die-offs of Alewife strew beaches with immense numbers of decaying fish, rendering the area unsuitable for human use and a health hazard. They can clog water intake pipes. Recovery of populations is rapid, almost sevenfold in 3 years. Die-offs are caused by sudden temperature changes from cold, deep to warm, shallow waters.
Cyprinidae - Carps and Minnows - Carpes et Ménés
Carps, minnows, shiners, daces, chubs and their relatives comprise 53 species in fresh waters across Canada and 22 in the NCR, 2 of which are exotic or introduced. Some species may enter brackish water but the family is primarily a freshwater one. The family is also found in Africa and Eurasia but is absent from South America, Madagascar, New Guinea and Australia. There are over 2100 species, almost 10% of the world's fishes.
Carps are small (under 5 cm) to large (up to 3 m) fishes characterised by throat or pharyngeal teeth in 1-3 rows, with a maximum of 8 teeth in a row, there are no jaw teeth, lips are usually thin and not sucker-like, the upper jaw is bordered by the premaxillae bones, barbels are present in 0-3 pairs (mostly 1 pair or none in Canadian species), 3 branchiostegal rays, no true stomach, cycloid scales, and there are no true fin spines (some species have a hardened, unbranched ray in the dorsal and/or anal fin). The 4 anterior vertebrae are modified into Weberian ossicles which connect the swimbladder, in effect a sounding board, to the inner ear. Carps have extremely sensitive hearing and this is thought to account for their success. Carps produce an "alarm substance" when injured. This chemical stimulates other carps to flee and hide, another useful adaptation.
Pharyngeal tooth counts are an important diagnostic feature. These teeth lie on a modified, fifth gill arch which can be seen or probed behind the shoulder girdle, just inside the gill opening. The arch has to be removed with dissecting equipment to count the teeth. Tooth counts are presented as a formula such as 2,5-4,1 which indicates 2 teeth in the outer left row, 5 in the inner left row, 4 in the inner right row and 1 in the outer right row. Teeth may be lost from major or minor rows so variant formulae are given after the principal one. A horny pad on the underside of the basioccipital bone of the skull is used to masticate the food against. Tooth form varies with the food - molar-shaped teeth are used to crush molluscs, flat but grooved surfaces for grinding plant food and sharp edged teeth for slicing various invertebrate foods. Fin ray counts are also important in identification. The dorsal and anal fins have 3-4 unbranched rays followed by several branched rays. The first 2-3 unbranched rays are short or close together and may be difficult to see. The branched rays are usually well-separated and obvious. The last 2 branched rays are traditionally counted as 1 ray since they articulate with a single basal bone in the supporting skeleton. North American scientists give counts of dorsal and anal fin rays to include the last unbranched ray (i.e. as 1 ray) plus all branched rays with the last 2 counted as 1. In this website I have given only branched ray counts for dorsal and anal fins with the last 2 counted as 1.
Carps are remarkable for changes they undergo during the spawning season. Some fish, which are usually silvery, develop bright reds and yellows. Nuptial, pearl or breeding tubercles develop on the head, scales and fin rays often in distinct patterns, and there are in some species swellings of the head or fin rays. These changes are most apparent in males. Tubercles and swollen rays are used to clasp females during the spawning act. Tubercles are also used to fight other males and defend and clean nests. Colour attracts females for mating. Generally males have longer pectoral fins than females. Nest building males are larger than females, the reverse of the situation in most fishes where egg-bearing females are the largest. Not all species build nests and some simply broadcast eggs over weed, gravel or sand. Fractional spawning is common in carps. This is a prolonged spawning season which ensures no single batch of eggs is lost to unfavourable, temporary environmental changes such as floods. Carps are mostly omnivores, feeding on small crustaceans, insects and some minute plants but some specialise in eating large plants, or other fishes. Diet is reflected in pharyngeal tooth shape as mentioned above. Gut length is important too. A long intestine indicates a reliance on plant material which takes longer to digest. A simple, s-shaped gut is found in insectivorous fish. A black peritoneum is thought to protect gut bacteria from damaging light. The bacteria aid in breaking down the strong cell walls of plants. Size and shape of the mouth are also indicative of diet. Carps are found in many diverse habitats from swift, cold streams to warm bogs. These are schooling fishes, especially when young.
Carps play an important role in freshwaters as food for other fishes and some species are commercially important as bait fish, as sport fish or as food in Asian countries. Raising minnows (smaller Carp family members) as bait and as forage fish for sport fish is a big business in the U.S.A. It should be noted that it is prohibited to use live fish as bait in Québec or to carry them in from the Ontario side of the Ottawa River (Bergeron, 2005). They are an important element in the commercial aquarium trade and certain species are used in experimental studies by scientists.
The :Lake Chub (Couesius plumbeus) (Agassiz, 1850) is widely distributed in areas all around the NCR but has not been caught within this area. The Blacknose Dace (Rhinichthys atratulus (Herman, 1804)) is also not uncommon east and west of the NCR and, like the previous species, has been mapped within the NCR (Bernatchez and Giroux, 2000) but there are no confirmed records.
Goldfish / Carassin
Carassius auratus (Linnaeus, 1758)

Taxonomy
Other common names include Golden Carp and Cyprin doré.
Key Characters
This species is identified by having 14-20 dorsal fin branched rays, a serrated spine at the dorsal and anal fin origins and no barbels, unlike the similar carp.
Description
Anal fin branched rays 5-6, usually 5, pectoral rays 14-17 and pelvic rays 8-10. Lateral line scales 25-34. Gill rakers 37-50. Pharyngeal teeth 4-4, with one tooth conical and the others with expanded crowns. Breeding males have small nuptial tubercles on the operculum, back and pectoral fin rays. The gut is elongate with several loops.
Colour
The golden or orange colour of artificially bred aquarium Goldfish is distinctive. However populations in the wild, if they breed successfully, gradually revert to a wild-type of colour. Golden fish are readily seen and eaten by birds and other fishes. Wild-type colour is an overall olive-green fading to a white belly. Young Goldfish are green, brown or bronze to almost black. Peritoneum dusky to black.
Size
Reaches 62.0 cm and 4.15 kg, possibly 5.0 kg; larger fish being hybrids with the Common Carp.
Originally found in China and Korea, now widely introduced in Canada by design or accident. Reported from British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, New Brunswick, Nova Scotia and Newfoundland. They have been found in North America for over 100 years.
Origin
This species is an exotic or introduced species in the NCR, presumably by aquarium releases.
Habitat
Goldfish appear to favour ponds, or pools in streams, with aquatic vegetation but are often introduced into small bodies of water as ornamental fish or out of curiosity to see if they will survive. They are tolerant of turbidity, polluted waters, winterkill conditions and very high temperatures (upper lethal limit 41.4°C, preferred temperature 27.9°C).
Age and Growth
Life span, at least in captivity, is 30 years or more but about 7 years is more normal in the wild. Sexual maturity is attained at 9 months to 4 years. Males are smaller and grow more slowly than females. In any population females outnumber males as much as 7:1.
Food
Food includes aquatic insects, crustaceans, molluscs, worms, detritus and plants. Goldfish have a palatal organ on the roof of the mouth used to taste and sort food.
Reproduction
Spawning occurs in warmer summer months at temperatures above 16°C and eggs are shed over plants or willow roots, often on sunny mornings. Each female is accompanied by 2 or more males and chases are reported with much splashing. Eggs are adhesive and up to 1.6 mm in diameter. A female will spawn 3-10 batches of eggs at 8-10 day intervals, with up to 4000 eggs being laid in each batch. Total fecundity is 380,000 eggs. Population numbers in confined areas are limited by a chemical released by the Goldfish which represses more spawning. Some populations of Goldfish are all-female clones.
Importance
Goldfish are familiar aquarium pets with many exotic varieties having been developed. These include Comets, Veiltails, Fringetails, Telescopes, Lionheads, Orandas, Shubunkins and others, often with bizarre shapes. They are also extensively used as an experimental fish, perhaps more than any other fish species. Studies include physiological and biochemical work and toxic chemical bioassays. Carp-goldfish hybrids have been used to demonstrate pollution in the Welland River near Niagara Falls. Fish from industrial areas had a higher frequency of cancers than those from a rural area. This hybrid is, therefore, a "sentinel" species or an advance warning system of deteriorating environmental conditions. Some are caught and sold with Common Carp as food.
Spotfin Shiner / Méné bleu
Cyprinella spiloptera (Cope, 1867)
Taxonomy
Other common names include Silver-finned Minnow, Satin-finned Minnow, Blue Minnow, Steelcoloured Shiner and Lemonfin Minnow. Formerly in the genus Notropis.
Key Characters
This species and other shiners (genera Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. This species is separated from its relatives by the last 2-4 membranes of the dorsal fin having black stripes.
Description
Dorsal fin branched rays usually 7, occasionally 6, anal fin branched rays 6-8, pectoral rays 12-16 and pelvic rays 7-9. Lateral line scales 34-41. The short gill rakers number 8-10. Pharyngeal teeth 1,4-4,1 or 1,4-4,0. Males have large nuptial tubercles on top of the head back to the dorsal fin, on the snout and on the lower jaw in a single row. Small tubercles are present on posterior edges of scales, particularly over the anal fin, and on pectoral, pelvic, caudal and anal fin rays.
Colour
The back and flanks are silvery to bluish or olive and the belly white or silvery-white. Flank scales are outlined with pigment. There is a narrow, olive stripe on the rear half of the body positioned below the lateral line. There is a mid-dorsal stripe passing on each side of the dorsal fin. Pectoral, pelvic and anal fins yellowish, especially in breeding males. Breeding males have an olive-yellow snout, steel-blue back, milky-white fin edges and the whole dorsal fin is blackened. Peritoneum silvery with some darker speckles.
Size
Reaches 12.0 cm.
Found from Québec City west through the Great Lakes (but not in Lake Superior) and southern Ontario to North Dakota and south to Alabama and Oklahoma. Coad (1987b) documents occurrence in the NCR.
Origin
This species entered the NCR from a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
Spotfin Shiners are usually found in rivers and larger streams over sand and gravel but may also occur in lakes. They are said to be tolerant of turbidity, siltation, high temperatures (up to 35°C, preferred temperature 29.5°C.) and pollution.
Age and Growth
Life span is 5 years and both sexes mature at 1 year, sometimes at 2 years of age.
Food
Food is mostly aquatic and terrestrial insects and crustaceans, with some plant material and even young fish. They eat eggs of their own species if these are deposited outside a crevice. Peak feeding occurred at 9 p.m. in a Michigan study.
Reproduction
Spawning takes place from May to September in Wisconsin but each locality has a more restricted peak season. Up to 7474, 1.2 mm diameter, adhesive eggs are deposited on the undersurface of logs or roots, under log bark or in crevices., or on vertical substrate Males defend a spawning territory of 50 cm or more which includes a crevice. They have been seen dragging rivals away by a grip on the pelvic or anal fin when an erected fin display failed to drive away the other male. Each male may grab the other and circle around at increasing speed until they are a blur in the water. Males make a "display pass" over crevices, swimming slowly and sometimes undulating or vibrating rapidly. Passes may occur up to 30 times before spawning. This usually attracts a female or the male may approach a school of females and hustle one toward the crevice. A male apparently presses a female against a log or crevice by positioning himself with his ventral surface touching her back, and both vibrate as eggs and sperm are shed. This can be repeated 2-3 times. When spawning on the undersurface of a log the male-female position is the same but they are upside down. Unusually for Carp Family members, the male of this species makes purring noises during courtship which are believed to be recognition signals. Spawning occurs fractionally, up to 12 times at intervals of 1-7 days. One female is reported to have laid 31 groups of eggs, each group having 10-97 eggs. Crevice spawning protects the eggs from predators, abrasion, smothering, displacement, and sunlight. Eggs laid in crevices have a better chance of being fertilised in the restricted space than those released into a current. Fractional spawning enables fecundity to be increased as not all eggs mature at the same time in a small body cavity. In addition, in crevice spawners a limited availability of crevices would restrict spawning activity unless crevices are used more than once. Fractional spawning also insures against loss of a generation to a sudden disaster.
Importance
This species has no specific importance in Canada but has a bait fish potential.
Common Carp / Carpe
Cyprinus carpio Linnaeus, 1758
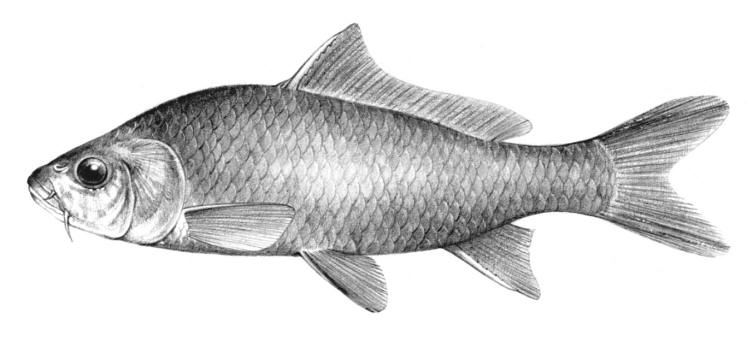



Taxonomy
Other common names include German, European, King, Mirror or Leather Carp; German Bass, Buglemouth Bass and Carpe allemande.
Key Characters
This species is characterised by having an elongate dorsal fin with 15-23 branched fin rays, spine-like rays near the dorsal and anal fin origins, 2 pairs of upper jaw barbels unlike the similar Goldfish, 21-27 gill rakers and 3 rows of pharyngeal teeth.
Description
The dorsal fin has the last unbranched ray developed as a toothed spine. The anal fin has a similar spine and 4-6 branched rays. Pectoral fin rays 14-19 and pelvic rays 8-9. Lateral line scales 32-41, when present. Scales may be absent (Leather Carp) or restricted to a few, enlarged scales (Mirror Carp) but Canadian fish are usually fully scaled. Pharyngeal teeth usually 1,1,3-3,1,1 and molar-like. Breeding males have fine tubercles on the head and pectoral fins.
Colour
The back is olive-green, yellowish-brown or grey, the flanks gold or bronze to silvery and the belly yellowish-white. Fins are dusky olive except the anal fin and lower caudal fin lobe may be reddish to orange and have a dark spot at their base. Scales on the upper flank have dark margins and bases. Peritoneum dusky. Faber (1984c) illustrates a larva.
Size
Reaches 128.0 cm and 44.9 kg, possibly 150.0 cm and 69.6 kg. The world, all-tackle angling record from France in 1987 weighed 34.35 kg. A 17.5 kg carp measuring 99 cm long is recorded from the Ganaraska River, Ontario but fish over 23 kg are known from Ontario. A 36.48 lbs (16.56 kg) carp from Smiths Falls, south of the NCR, ranked twelfth in the 1998 Northeastern Bowfishing Championship (Bouchard in Kerr, 1999b). A 25 lbs (11.35 kg) carp, 3 feet (91.4 cm) long and 23½inches (59.7 cm) girth, was caught in Dows Lake of the NCR in April 1962 (Anonymous, 1962a), and a fish about 26 lbs (11.8 kg) in the Rideau River in 2002 (Sevitt, 2002).
Found in New Brunswick, Québec, Ontario, Manitoba, Saskatchewan and British Columbia from introductions. First introduced to Canada in the late nineteenth century, as early as 1880 in Ontario. Also introduced world-wide in suitable waters. The native distribution is from eastern Europe to western China.
Origin
The Common Carp is an introduced species to the NCR. Dymond (1939) did not report this species in his Fishes of the Ottawa Region but McCrimmon (1968) recorded it from Pointe Gatineau in 1944. First reported from the Rideau River system in the 1950s (Phelps, 2001). Large Carp are easily seen in the Rideau Canal in summer and in Brown's Inlet in the spring.
Habitat
The Common Carp is found in lakes, canals and marshes and slow-moving rivers, and possibly even some sewers in the Ottawa River
drainage (http://mywebpage.netscape.com/thewizardoffish/2.htm, downloaded 7 June 2003). Carp avoid fast water in streams. A fish kill
involving this species was noted at Mud Lake, Britannia Woods on 12 April 1984 probably from oxygen depletion under ice (Halliday,
1985) and again on 16 April 2003. A fish kill along extensive stretches of the Rideau River and Canal and in Mackay Lake, Rockliffe
 Park was reported in spring 1956 (newspaper reports).
Temperatures up to 35.5°C are tolerated and their preferred temperature is 29.7°C.
Below about 4°C carp are usually inactive. Carp tolerate turbid and low oxygen conditions. They can often be seen basking at the
surface or feeding on algae and their dorsal fins break the water surface. Carp also leap from the water but the reason is unknown.
Large fish often move into shallows in the afternoon and evening. They retreat to deeper water in winter but rarely descend below 30 m
in lakes. The construction of the Carillon Dam created major impoundments and favourable conditions for carp in the Ottawa River
although Qadri and Rubec (1974) found numbers in the river at Ottawa to be relatively low.
Park was reported in spring 1956 (newspaper reports).
Temperatures up to 35.5°C are tolerated and their preferred temperature is 29.7°C.
Below about 4°C carp are usually inactive. Carp tolerate turbid and low oxygen conditions. They can often be seen basking at the
surface or feeding on algae and their dorsal fins break the water surface. Carp also leap from the water but the reason is unknown.
Large fish often move into shallows in the afternoon and evening. They retreat to deeper water in winter but rarely descend below 30 m
in lakes. The construction of the Carillon Dam created major impoundments and favourable conditions for carp in the Ottawa River
although Qadri and Rubec (1974) found numbers in the river at Ottawa to be relatively low.
Age and Growth
Life span may reach 47 years with males maturing generally at 2-4 years and females at 3-5 years. Most fish live 9-16 years. Growth rates vary markedly, even in adjacent waters.
Food
Mouthfuls of bottom ooze are taken up, spat out and the food items selected. These include aquatic insects, crustaceans, worms and molluscs, and more rarely, fish. Plant material is ground up by the molar pharyngeal teeth and includes algae, seeds, wild rice, leaves and various aquatic plants. Organic sewage is also eaten. Some surface feeding on algal mats or insects will also occur. A wide variety of other fishes and birds eat smaller carp as do predatory aquatic insects, frogs and toads. Carp eggs are eaten by other family members, catfishes and sunfishes. Adult carp are too large for most predators except parasitic lampreys.
Reproduction
Spawning occurs in groups of 1-3 females and 2-15 males in shallow, weedy waters. Temperatures of 17°C or warmer are necessary and the season lasts several days to several weeks. Spawning may occur any time between May and August in Canada and is easily observed with some fish having their backs out of the water and much audible splashing. Eggs adhere to vegetation and are about 2.0 mm in diameter. A large female can contain 2,208,000 white to yellowish eggs. Eggs hatch in 3-12 days, depending on temperature, and larvae are 3.0-5.0 mm long.
Importance
An exotic species, Common Carp are a nuisance because they uproot vegetation used by native species for cover, food and spawning. This activity also increases water turbidity to levels which many native species cannot tolerate. Stirred up silt may also smother eggs of native species. Carp also compete with Largemouth Bass and other species for food. Various methods have been employed to eradicate carp from netting to poisons and electric shockers, usually without success except in the smaller, enclosed ponds and lakes.
In the U.S.A. it is sought by anglers but is not fished for as extensively in Canada although tours from England to Ontario are now available for anglers. Carp are a premium game fish in England with angling societies, newsletters, specialised rods and other gear devoted to this species. Some carp are caught in Canada using bow-fishing, spears or spear-guns and scuba gear. In many parts of the world, carp are raised in ponds and are an important food fish. Commercial fisheries for carp above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b). A commercial fishery in Ontario has taken up to 454,000 kg annually valued at $100,000. The total Canadian catch in 1988 was 780 tonnes. The fish are sold alive, fresh or smoked and are often baked. In recent years carp have been found to harbour high levels of the toxic chemicals PCBs, and in Wisconsin it is recommended that carp not be eaten more than once a week. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Rideau River and South Nation River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Brightly coloured varieties of carp are known as "koi" and are kept as ornamental fish. Colours include red, orange, white, black, blue and yellow in various combinations.
Brassy Minnow / Méné laiton
Hybognathus hankinsoni Hubbs, 1929
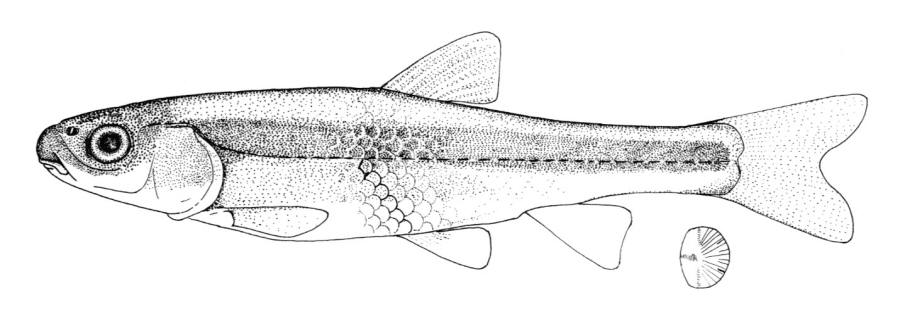
Taxonomy
Other common names include Grass Minnow and Hankinson's Minnow.
Key Characters
This species resembles the shiners (genera Cyprinella, Luxilus, Notropis) but has an elongate intestine which has coils on the right, and a subterminal mouth with a diagonal fold running past its corner. It is distinguished from its relative, the Eastern Silvery Minnows, by the rounded dorsal fin, brassy colour, no thin black line along flank and 14-22 radii on scales in the adult.
Description
Dorsal fin branched rays 6-7, usually 7, anal branched rays 5-8, usually 7, pectoral rays 13-15 and pelvic rays 8. Lateral line scales 35-41. Pharyngeal teeth 4-4 with a flat, oblique grinding surface. Males have breeding tubercles on rays 2-8 of the pectoral fin in multiple rows (5 or more distally).
Colour
The back is olive-green to brown with a brassy sheen, the flanks are brassy to dull silvery and the belly cream-white. There is a mid-flank stripe which is best developed from below the dorsal fin posteriorly (and best seen in preserved fish). Scales above the stripe are darkly outlined and form 2 indistinct, zig-zag stripes. There is a mid-dorsal stripe on the back. Dorsal, anal and caudal fins are yellowish and rays are outlined by dark pigment. The dorsal fin membranes are lightly spotted. The anterior pectoral and pelvic fin rays are outlined with dark pigment and the rest of these fins is clear. Some fish have a faint spot at the caudal fin basin. Breeding males become more brassy and fins take on a brassy tinge. Peritoneum jet black to dusky black.
Size
Reaches 15.8 cm standard length.
Found in southwestern Québec, southern Ontario, western but not northern or eastern Lake Superior drainages, southwestern Manitoba, Milk River basin of southwestern Saskatchewan and southeastern Alberta, the Peace River basin in northwestern Alberta and the Athabasca River basin in northeastern Alberta, and in the lower and upper Fraser River and the upper Peace River in British Columbia. In the U.S.A. from New York to Montana and south to Kansas.
Origin
This species entered the NCR from a Mississippian refugium or possibly a Missourian refugium (Mandrak and Crossman, 1992).
Habitat
Brassy Minnows are found in dark, acidic ponds, shallow lakes and small, slow streams which have silt bottoms. Such areas have few or no predators. In the NCR has temperatures of 17-20°C in May-June in these habitats. Some fish are found over sand, gravel, stones or bedrock.
Age and Growth
Maturity may be attained at 1-2 years with some fish reaching 5 years of age.
Food
Food is bottom ooze for the algae, bacteria, protozoa and minute crustaceans, and some aquatic insects. Up to 94% of the food is the algae, such as diatoms and desmids. Plankton may also be taken. Feeding occurs in schools with a peak at 1-3 p.m. in one study.
Reproduction
Spawning probably takes place in May-July in Canada in marshy areas. Eggs are shed at 10°C or higher. Eggs are up to 0.8 mm in diameter, yellow and number 2500. In Wyoming spawning occurred between 11 a.m. and 5 p.m., peaking at 2 p.m., in and over vegetation. Males and females aggregate in schools numbering in the thousands. One to 15 males approach a female at the edge of the school and she would respond in one of two ways. She may spiral up and leap out of the water, discouraging the males, or swim to vegetation which would stimulate one or more males to press against her, quiver and release eggs and sperm. The vibrations stir up sediment.
Importance
This minnow is used as bait in the U.S.A. Its distribution in Québec is limited and it could become threatened or vulnerable (www.fapaq.gouv.qc.ca, downloaded 7 October 2002).
Eastern Silvery Minnow / Méné d'argent
Hybognathus regius Girard, 1856
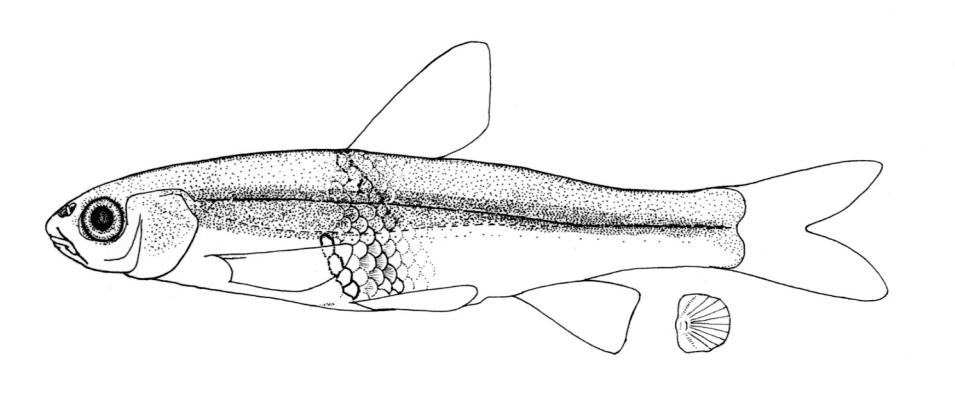
Taxonomy
Another common name is Méné bleu and Méné d'argent de l'est. This species was formerly thought to be the same as the Western Silvery Minnow and both were combined with the Central Silvery Minnow (not in Canada) under the name Hybognathus nuchalis Agassiz, 1855.
Key Characters
This species resembles the shiners (genera Cyprinella, Luxilus, Notropis) but has an elongate intestine which has coils on the right, and a subterminal mouth with a diagonal fold running past its corner. It is distinguished from its relative, the Brassy Minnow, by its falcate dorsal fin, silvery colour, a thin black line along flank (partly over the mid-flank stripe) and 12 or less radii on adult scales below the dorsal fin.
Description
Dorsal fin branched rays 7, anal branched rays 7-8, pectoral rays 14-16 and pelvic rays 7-8. Lateral line scales 38-40. Pharyngeal teeth 4-4 with a flat, grinding surface. Males have breeding tubercles on the head, back, flank scales, particularly abundant on anterior scales, and on both sides of all fins, best developed on the upper pectoral and pelvic fins.
Colour
The back is olive with the flanks silvery and the belly silvery-white. There is a broad stripe along the mid-line of the back. The mid-flank stripe has a sharp upper edge and begins half way between the head and the level of the dorsal fin origin. It extends to the base of the tail fin where it is widened or spot-like. A thin black line runs through the middle of this stripe and then ascends slightly. The preorbital bone of the head has a silvery spot. The lower margin of the caudal fin is white. Dorsal and caudal fin rays and the rear edges of anal rays 2 and 3 are outlined with pigment. Pectoral fin rays 1-7 are also outlined with pigment but the rest of the fin is clear. Pelvic fins clear. Breeding males become yellowish on the flanks and lower fins. Peritoneum black.
Size
Reaches 15.7 cm total length.
Found in Lake Ontario drainages, the lower Ottawa and upper St. Lawrence river basins of Ontario and Québec south to Georgia east of the Appalachian Mountains.
Origin
This species entered the NCR from an Atlantic coastal refugium.
Habitat
This species is common in lakes and streams where there are pools and backwaters. The bottom is variable and includes ooze, sand, gravel and boulders. It is a schooling species and is sensitive to turbidity and siltation but occurs where there is availability of detrital food (Mandrak and Ramshaw, 1998).
Age and Growth
Life span is 3 years. Males are mature in their second year, females spawn at 1 year of age in New York.
Food
Food includes bottom ooze and algae such as diatoms, although in Lac Memphrémagog, Québec 0+ fish fed on cladocerans principally, with rotifers and chironomids, switching to almost entirely organic detritus with increased age (Mandrak and Ramshaw, 1998).
Reproduction
Spawning occurs in late April to mid-May in New York at about 13-21°C, starting at 10:00 a.m. and lasting until 4:00 p.m. In Delaware, spawning takes place in April and May at 10-20°C. Females may contain 6600 eggs. The spawning behaviour in New York commences with a concentration of males inshore with females in deeper water. A female ready to spawn moves towards shore and is escorted by 1-10 males. A female may jump out of the water and return to deeper water without spawning. Usually 2 males flank her and the fish quiver strongly in very shallow water (5-15 cm deep). Non-adhesive, demersal eggs of 1.0 mm diameter are shed onto the ooze bottom. Spawning activity stirs up the ooze bottom. The female returns to deeper water and the males remain to wait for another female.
Importance
This species has been used as a bait fish in Ontario and in Québec for Yellow Perch, Northern Pike and Walleye in winter. About 15,600 kg has been caught around Montréal in the fall and Toner (1943) notes considerable in the Ottawa River. This species was placed in the "Not at Risk" category in 1997 by the Committee on the Status of Endangered Wildlife in Canada.
Common Shiner / Méné à nageoires rouges
Luxilus cornutus (Mitchill, 1817)
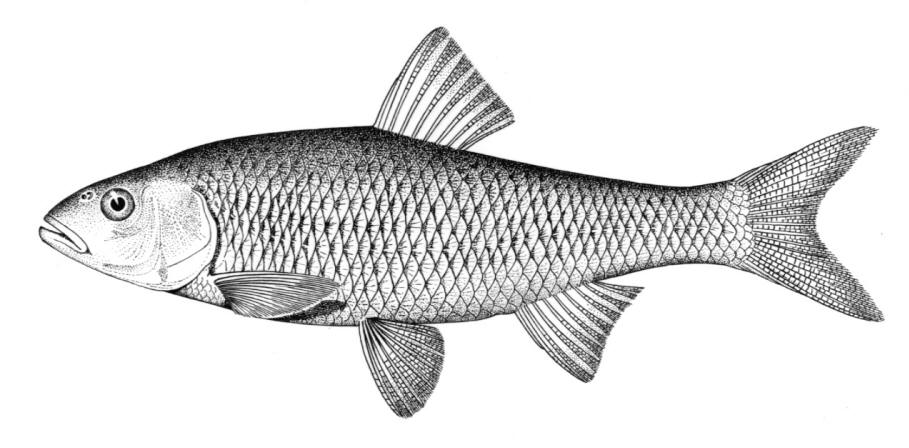
Taxonomy
Other common names include Eastern Shiner, Redfin Shiner, Silver Shiner, Silverside, Rough-head, Hornyhead, Creek Shiner, Dace, Skipjack and Méné à ruisseau. Formerly in the genus Notropis.
Key Characters
This species and other shiners (genera Cyprinella and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is distinguished by having anal fin branched rays 7-9, usually 7-8, exposed part of anterior lateral line scales twice as high as wide, dorsal fin origin over or in front of pelvic fin insertion level, predorsal scale rows 16-30, usually 18-24 (counted from below dorsal fin origin to head, 3-6 scale rows up from the lateral line), and the chin is not pigmented.
Description
Dorsal fin branched rays 7, pectoral rays 15-17 and pelvic rays 8-9. Lateral line scales 34-44. Short gill rakers number 9-10. Pharyngeal teeth hooked at the tip, usually 2,4-4,2 with such variants as 2,4-4,0 or 1,4-4,1. Males have nuptial tubercles on the snout, in a single row on each lower jaw, sparsely on the nape back to the dorsal fin including the first ray and on the anterior pectoral fin rays.
Colour
The back is olive-green to olive or bluish-olive with a purple to grey-blue mid-dorsal stripe, flanks are silvery with bronze tinges and some darkened scales, and the belly is silvery-white. The flanks bear a dark stripe bordered with lighter stripes above and below. There are 3-5 grey-blue, parallel wavy lines on the upper flank but these do not meet in a V behind the dorsal fin. Upper flank scales are not outlined with pigment. Fins are clear. Young are more silvery and lack the bronze tinges. Breeding males have pink to red on the distal third of all fins, the anterior flanks and head are pink and there is a golden stripe on the upper flank. The head is darkened to a lead blue. Peritoneum black to brown. Faber (1984c) illustrates a larva.
Size
Reaches 20.8 cm.
Found from Nova Scotia and New Brunswick through southwestern Québec and southern Ontario (rare in lower James Bay tributaries) and the Great Lakes but not the northeastern shore of Lake Superior, to southern Manitoba and southeastern Saskatchewan. In the U.S.A. south to Virginia and Colorado.
Origin
This species may have entered the NCR from either a Mississippian or an Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
Common Shiners are found abundantly in streams, rivers and less frequently at lake margins. The water may be clear or cloudy, the bottom may vary from boulders to mud, silt and detritus, and the current varies from still to medium. Their preferred temperature is 21.9°C.
Age and Growth
Life span in Québec is about 7 years, perhaps up to 9 years elsewhere, with maturity attained at 1-3 years. Males grow faster than females and in the Laurentides, Québec study were 1.8 cm longer at age 5 and weighed more than twice as much. This population matured at 3 years. However growth was slower than in eastern Ontario and U.S.A. fish.
Food
Food is taken from the surface and the bottom and includes aquatic insects, algae, other plants, crustaceans, worms, protozoans, desmids, and small fishes. As much as 71% of the food may be plant material. These fish have been characterised as roving opportunists, taking whatever food is available. Fish thought to be this species have been observed taking orthopterids and leafhoppers from the water surface in the South Castor River of the NCR by F. W. Schueler (in litt., 11 August 2004). They are food for various other fishes and birds.
Reproduction
Spawning takes place by day over stream riffles from May to July when water temperatures exceed 16°C. Tubercle scars, indicating spawning had ended, were found on NCR fish caught on 25 July (McAllister and Coad, 1975). In the Laurentides, Québec spawning occurs near the end of May and the beginning of June. A nest may be excavated in gravel beds of streams or the nests of other species may be used. Males clean a nest area by using their heads to move stones. Each male defends his nest site against other males using his head tubercles to butt opponents. Fin raising may scare away an opponent or pairs of males may parallel swim for up to 2 m with the caudal peduncle and tail slightly raised, followed by butting. The winner returns to the nest site. Males "tilt" to attract a female onto the nest site, inclining the body to one side then the other. The female takes up a position on the side of the male which makes the acutest angle with the bottom. Males may aggregate in masses of 100 fish, all fighting, butting, biting and chasing. Spawning occurs on the fourth day after the aggregate forms. Injuries often result and become infected with fungus. A dominant male can spawn 8 times in 5 minutes with 36 courting tilts. He may occupy an outlying area from the aggregate association, using the area for only a day or two at a time. Once the female moves onto the nest site, the male curves around her with his pectoral fin under her breast and his caudal peduncle over her back, and squeezes so eggs and sperm are shed. The female darts forward abruptly after the spawning clasp and often breaks the water surface. This process is repeated many times as less than 50 eggs are shed during each spawning. Hybrids are common because other species' nests are used, notably with the Creek Chub, River Chub, Hornyhead Chub, Fallfish, Central Stoneroller and the Rosyface Shiner. Eggs are about 1.5 mm in diameter and orange when laid, and adhere to the gravel. Each female can have up to 3940 eggs. Larvae are 5.0-6.0 mm long at hatching.
Importance
This species is often used as a bait fish for Northern Pike and Walleye. It will rise to a dry fly and is said to be an acceptable food item.
Pearl Dace / Mulet perlé
Margariscus margarita (Cope, 1867)
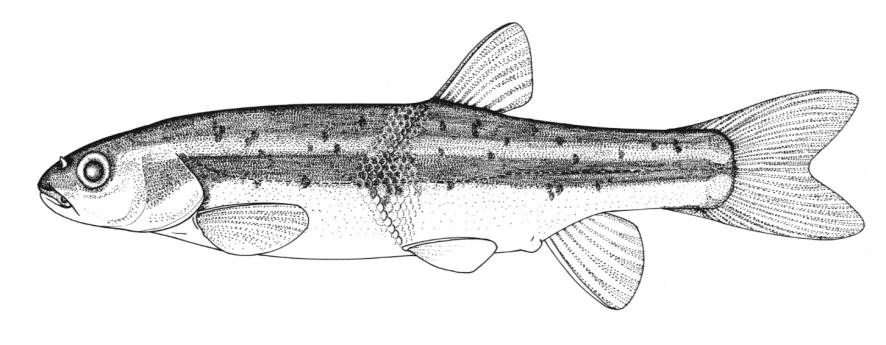
Taxonomy
Other common names include Nachtrieb Dace, Northern Pearl Dace, Northern Minnow and Northern Dace. Formerly placed in the genus Semotilus.
Key Characters
This species is distinguished by having protractile premaxillae (a groove between the upper lip and the snout), a flat barbel in advance of the mouth corner in the groove above the upper lip, lateral line scales 46-79 (usually 65-75) and no black spot at the dorsal fin base. The barbel may be absent or very small on one or both sides in which case the high scale count in a complete lateral line, short s-shaped gut, silvery peritoneum, small mouth and lack of unique characters found in other Carp Family members distinguish this species.
Description
The upper jaw does not reach back to a level with the front of the eye. Short gill rakers number 4-6. Pharyngeal teeth hooked, 2,5-4,2, 2,4-4,2, or 2,4-4,1. Dorsal fin branched rays 6-7, usually 7, anal fin branched rays 6-7, usually 7, pectoral fin rays 14-19 and pelvic rays 8-9. Males have large nuptial tubercles on up to 8 pectoral fin rays in a double row, with a few, very small ones on the head, lining scale edges, and densely tuberculate on the first 2 branchiostegal rays. Tubercles are also present on other fins, varying greatly in number and extent. Pectoral fins are longer and wider in males than in females.
Colour
The back is dark brown to almost black, greyish or green, the flanks are olive to silvery and the belly silvery-grey to white. Flanks may have dark scales. Young fish have a brown-black stripe ending in a spot at the tail base. Breeding males and some females are orange-red below the mid-flank. This colour persists from fall to the spawning season. Peritoneum silvery with some darker speckles dorsally.
Size
Reaches 15.8 cm.
Found from Nova Scotia and central Labrador west to Alberta, eastern British Columbia and the south of Great Slave Lake, N.W.T. In the U.S.A. south to Virginia, west to Montana with relict populations in South Dakota, Nebraska and Wyoming.
Origin
This species entered the NCR from an Atlantic, Mississippian or Missourian refugium (Mandrak and Crossman, 1992).
Habitat
Pearl Dace are found in clear streams, pools and channels of headwaters, bogs, ponds and small lakes. The bottom varies from bedrock to mud, with the latter preferred, and the water is usually still or slow moving. Their preferred temperature is 16.2°C. Pearl Dace produce an alarm substance from skin cells when injured. Other Pearl Dace react in 2 phases. The first phase is rapid, unpredictable swimming which serves to remove the fish from danger and the second phase is several hours of inactivity on the bottom which is thought to reduce the chance of detection by a predator after the first, confusing flight phase. Pearl dace are normally schooling mid-water fish. Numbers of this species in Lac LaPêche, Gatineau Park have declined while Golden Shiners have increased (Rubec, 1971).
Age and Growth
Life span is 4 years for females and 3 years for males in Maryland. Females grow faster than males in Québec and both sexes mature at 2 years.
Food
Food is aquatic and terrestrial insects, crustaceans, young fish, detritus and occasionally algae and larger plant debris.
Reproduction
Spawning occurs in late March to June at 16°C or more in clear, shallow water (perhaps half a metre deep) over sand or gravel in weak to moderate current. Spawning in Lake Gamelin, Québec occurred in the first half of May. A male defends a territory about 20 cm wide against other males. Ripe females are allowed into the territory or driven into it by the male. The male places his pectoral fin under the anterior part of the female's body, lifting it up at an angle of about 30°, and his caudal peduncle over that of the female, pressing her belly down. This may stretch the female's belly and help release the eggs. The pectoral fin tubercles of the male aid in grasping the female. The fish vibrate for about 2 seconds and eggs are shed. Females repeat spawning numerous times with different males. Each female may have up to 4240 eggs of 1.4 mm diameter.
Importance
It is used as a bait fish in Canada and anglers may catch this species on dry flies.
Hornyhead Chub / Tête à taches rouges
Nocomis biguttatus (Kirtland, 1840)
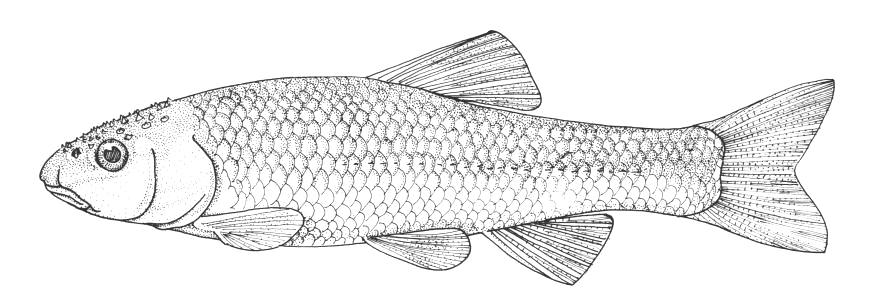

Taxonomy
Other common names include River Chub, Jerker, Horned Chub, Indian Chub and Redtail Chub.
Key Characters
This is the only member of the family in the NCR with a mouth corner barbel, large scales, and a terminal or nearly terminal mouth.
Description
Lateral line scales number 38-48, branched dorsal fin rays 6-7, usually 7, branched anal fin rays 5-6, usually 6, pectoral fin rays 14-17 and pelvic rays 8. The mouth is terminal or the snout may project a little over the mouth. Breeding males have up to 130 large and pointed tubercles on top of the head, as well as some on the pectoral fin rays. Pharyngeal teeth are 1,4-4,1, 4-4 or 4-3 and have hooked tips. Gill rakers are short and number about 12 total.
Colour
The back is olive-brown, flanks are silvery or iridescent green and the belly yellowish to white. There is a stripe along the middle of the back and along the flank and there is a round, black spot at the caudal fin base. The caudal fin is yellow in adults. Upper flank scales are outlined with pigment and have a dark bar at their base. Dorsal and anal fins are orange to reddish in breeding males and a strong, black flank stripe develops. A characteristic red spot is found behind the eye. The head and upper body have pink and blue tinges. Females also develop a flank stripe but this fades as they move away from the spawning area. Young fish have a characteristic red caudal fin and the anal and dorsal fins are also red. The peritoneum is brown to dusky with melanophores.
Size
Reaches 25.0 cm total length.
The occurrence of this species in the NCR is documented by Coad and Alfonso (2005). It was first found in the NCR in 2004 in the Jock River and its tributary Kings Creek. It is also recorded from the Jock River near the Prince of Wales Bridge, near Munster and near the McCaffrey Road Bridge (Greg Hutton, pers. comm., 3 March 2005).
Origin
This species is spreading from southwestern Ontario eastwards, whether by natural means or aided by bait fishing releases.
Habitat
This chub prefers slow, clear, gravel- or sand-bottomed streams and rivers. It is found in pools and riffles and may hide under rocks.
Age and Growth
Life span is 4 years with males growing faster than females and reaching a larger size since they build and defend a nest. Females mature at age 3 in Ontario.
Food
Diet includes vascular plants, filamentous algae, aquatic insects, worms, crayfish, snails and fishes. Plant material is probably not absorbed as the gut is short but associated animal material must be utilised.
Reproduction
Spawning takes place once water temperatures exceed 16ºC, usually in May to July. Males build a nest of pebbles and stones in shallow water, often below a riffle. the male moves stones by rolling them along using its head and mouth or by carrying them in its mouth. A nest can be 1 nm wide, 1 m long and 15 cm deep. A spawning tough is excavated on top of the nest. The male leads or drives females over the nest, presses the female's genital area into the trough with his caudal peduncle over hers, both fish vibrate, the female gapes, and eggs and sperm are shed. Eggs are adhesive. The male adds more pebbles to the nest to protect the eggs and excavates a new trough downstream of the first. Males may spawn with more than one female, both at the same time and consecutively. Each female contains up to 995 eggs of 2.0 mm diameter. Eggs are shed at intervals and each nest may contain eggs from up to 10 females. Both males and females raid nests to steal and eat eggs. Accessory males are found around nests, adding and removing pebbles. These are small, non-spawning chub males practising reproductive behaviour or smaller, late-spawning males waiting for the earlier spawning, large males to vacate the nest site. Ideal nest sites are limited and are also used by Common Shiners which drive away smaller species while the chub drives away larger species using its head tubercle to butt other fish. Bluntnose Minnows and Johnny Darters also use the nest site, among other species not found in the NCR.
Importance
This species was placed in the "Not at Risk" category in 1988 by the Committee on the Status of Endangered Wildlife in Canada. Anglers can catch this fish on baited hooks and dry flies and they are said to be quite tasty. They are use as bait for Northern Pike, Walleye, basses and catfishes.
Golden Shiner / Méné jaune
Notemigonus crysoleucas (Mitchill, 1814)
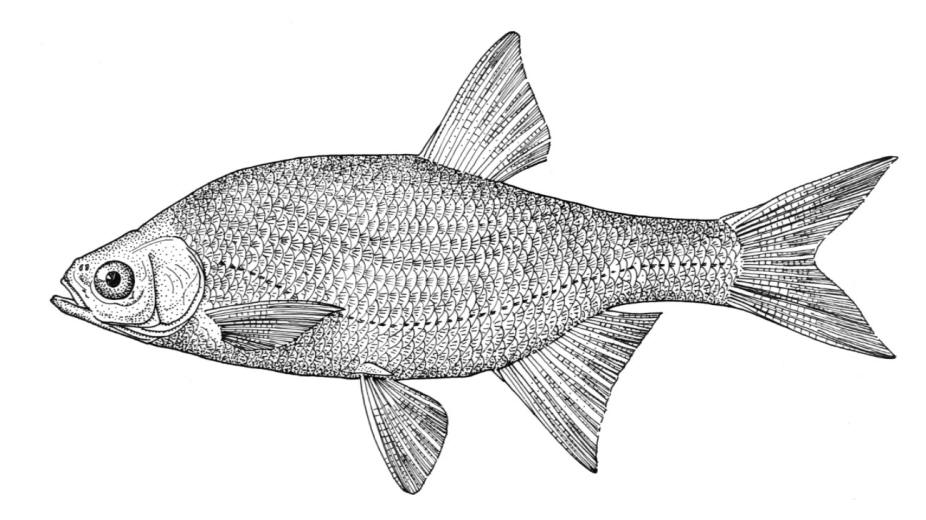

Taxonomy
Other common names include Chatte de l'est, Eastern Golden Shiner, Roach, Bream, American Roach, American Bream, Butterfish, Sunfish, Windfish, Goldfish, Dace, Chub, Bitterhead, Gudgeon, Young Shad, Pond Shiner, Méné plat and Petite laquaiche.
Key Characters
This species is identified by the strongly decurved lateral line with 39-57 scales, a naked, fleshy ventral keel between the pelvic fins and the anus on the belly mid-line and 7-18 (usually 11-13 in Canada) branched rays in the anal fin.
Description
Pharyngeal teeth 5-5 with a grinding surface and hooked tips. Dorsal fin branched rays 6-8, pectoral rays 15-18 and pelvic rays 8-9. Gill rakers 18-22. Males have nuptial tubercles on the scales with up to 15 tubercles lining each scale margin with an occasional 1-2 tubercles on the central part of the scale. The largest tubercles are on mid-flank below the dorsal fin. Much smaller tubercles are scattered over the top and sides of the head, tip of the upper lip, branchiostegal rays, central caudal fin rays and weakly on other fins. There is a patch of larger tubercles on the chin.
Colour
Overall colour golden or brassy with the back olive-green to dark brown. The belly is silvery-yellow. Fins yellowish. Young fish are more silvery and can be darkly pigmented around each scale. There is also a dark lateral stripe in young. Breeding males develop orange-red pelvic fins and a black edge to the anal fin. The nape swells to a hump. Peritoneum dusky to silvery with dark melanophores. Golden shiners hybridise with Rudd, an exotic species from Europe which is easily confused with this species. Faber (1984c) illustrates a larva.
Size
Reaches 30.5 cm. A 24.0 cm total length specimen from Lac Philippe in Gatineau Park is unusually large for Canada (Pluritec Ltée, 1982).
Found from P.E.I. (introduced) and Nova Scotia westward through southern Québec, the James Bay drainage at Lake Abitibi and the Great Lakes to southern and central Manitoba and southeastern Saskatchewan, and south to Florida, the Gulf states and Mexico. Widely introduced in the U.S.A. outside this range.
Origin
This species entered the NCR from possibly an Atlantic coastal or a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
Golden Shiners are found in large schools in clear, weedy areas, particularly in lakes but also in embayments of large rivers and in streams. They are often associated with branches and rocks or some other form of shelter. They are the dominant fish larvae in shallow bays with bulrushes in Heney Lake, Québec, just north of the NCR (Faber (1980). They move into deeper water as they grow. At an average length of 30 mm, they leave the littoral zone and live among submerged macrophytes offshore. These shiners favour temperatures in Canada in the low 20s°C but tolerating up to 34°C. The highest lethal temperature is 40°C, unusually high for a North American Carp Family member.
Age and Growth
Maturity is usually attained at ages of 1-2 years and life span may be 10 years. Females grow faster, larger and live longer than males. Growth varies with latitude but also variations within a similar climate zone are great.
Food
Food is principally crustaceans and flying insects taken in mid to surface waters. Other aquatic insects, molluscs, water mites, small fishes, phytoplankton and filamentous algae are also utilised. Usually only 2-3 items dominate in the diet and the dominant items vary with locality and season. Cladocerans and filamentous algae are recorded as particularly important in several studies. Shiners use 2 methods to forage on zooplankton. Large items like Cladocera (water fleas) are usually located and seized individually. Small, high density items are taken by pump filter-feeding. The shiner can switch between the 2 feeding modes as conditions dictate. This shiner may eat bass fry in ponds. Many sport fishes find this abundant shiner an important food source including Largemouth Bass, crappies and Muskellunge. The Northern Water Snake (Nerodia sipedon) eats this fish in Ramsay Lake, Gatineau Park (McMurray, 1984). A wide variety of birds also eat this shiner. In Ontario, population densities of this shiner are strongly influenced by predator densities. Food abundance does not appear to influence numbers of shiners.
Reproduction
Spawning takes place from May to August in New York when water temperatures exceed 20°C. Adhesive eggs are scattered over filamentous algae or other plants when 1-2 males chase and nudge a female. Yellow eggs are about 1.2 mm in diameter and a female can have up to 200,000. Larvae are 3.0-4.0 mm long at hatching. Largemouth Bass nests have been used by Golden Shiners as a spawning site. The male bass does not interfere and his presence serves to protect the eggs from predators.
Importance
This shiner is the leading bait fish in North America and is even pond cultured in the U.S.A. for bait sales or for food in fish hatcheries. A tolerance of low oxygen and high temperatures makes it a favourite for bait buckets. Around Montréal, 15,600 kg are caught each fall for bait. Some authors report it to be excellent eating.
Emerald Shiner / Méné émeraude
Notropis atherinoides Rafinesque, 1818
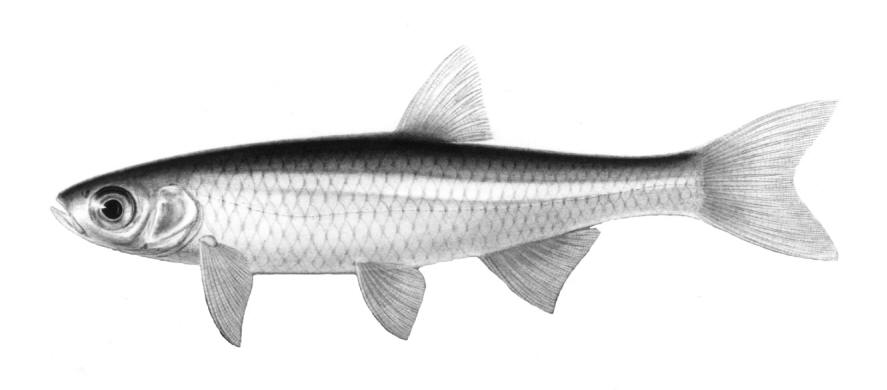
Taxonomy
Other common names include Lake Shiner, Lake Silverside, Buckeye Shiner, Milwaukee Shiner, Lake Michigan Shiner, Grands yeux, Vitreux and Épingle.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is separated from its relatives by having 8-12 branched anal fin rays, anterior flank scales rounded, dorsal fin origin behind the level of the pelvic fin insertion, slender body (body depth usually less than head length), snout length less than eye diameter, pectoral fin rays usually 15-17 and flank pigment ends above the lateral line.
Description
Dorsal fin branched rays 6-7, pectoral rays 13-17 and pelvic rays 8-9. Lateral line scales 35-43. Short gill rakers number 10-13. Pharyngeal teeth are hooked, 2,4-4,2, with occasional loss of teeth in both major and minor rows. Breeding males have tubercles on the upper pectoral fin rays 2-10 and minute tubercles on the head and sometimes the body.
Colour
The back is emerald green, blue-green to greenish-yellow and iridescent, flanks silvery and the belly is silvery-white. There is an emerald green or metallic silver mid-flank stripe and narrow mid-dorsal stripe on the back. Upper flank scales have dusky edges. Fins are clear except for white areas on the anal and caudal fins. The overall silvery colour is lost in preserved fish and mid-flank stripe is most apparent, best developed posteriorly. Peritoneum silvery and speckled brown.
Size
Reaches 12.4 cm standard length.
Found from southwestern Québec, through much of Ontario and Manitoba, throughout Saskatchewan, most of Alberta except the southwestern part, in the northwestern part of British Columbia and adjacent parts of the N.W.T. around Great Slave Lake and the upper Mackenzie River. In the U.S.A. south to the Gulf Coast west of the Appalachian Mountains.
Origin
This species entered the NCR from a Mississippian refugium, or possibly a Missourian refugium (Mandrak and Crossman, 1992). The presence of this species in Gatineau Park may be from discarded bait fish brought in by anglers (Rubec, 1975a).
Habitat
Emerald Shiners are found in large rivers or lakes where they school in open, surface waters. They overwinter in deeper water after moving inshore in the spring and in the fall. Their preferred temperature is 22-25°C and they are tolerant of turbid water.
Age and Growth
Life span is about 4 years with maturity as early as 1 year.
Food
Food is plankton, some insect larvae and surface insects, fish eggs and fry, worms and algae. There may be a migration with the plankton as it rises to the surface in the evening and descends in the morning. These shiners are an important food source for a wide range of predators including Lake Trout, Smallmouth Bass, Rainbow Trout, Northern Pike and various surface-feeding birds.
Reproduction
Spawning occurs in June to August in Canada, probably at temperatures over 20°C. In Wisconsin the spawning season may run from May to August, peaking in June-July. Lake Erie spawners gather offshore in schools over gravel shoals numbering in the millions at about 2-6 m. Eggs sink to the bottom and are not adhesive. A male appears to pursue a female for a few seconds until he overtakes her, presses against her perhaps with interlocked pectoral fins, the pair arch upward and roll, releasing eggs and sperm. Females may spawn more than once in a season. Eggs are up to 0.7-0.9 mm in diameter and mature yellow eggs average 3410 for all age classes in Lake Simcoe, Ontario to as high as 8733 eggs for an age 3 fish. Some females survive to spawn in their fifth summer.
Importance
This is the most important bait fish because of the immense schools which can be caught commercially. It is even pickled and sold preserved in jars as bait, especially as it is difficult to keep alive. In Québec, despite its abundance, it is relatively little used, except in fall for the winter fishing season, because of its fragility in captivity and on the hook.
Blackchin Shiner / Menton noir
Notropis heterodon (Cope, 1865)
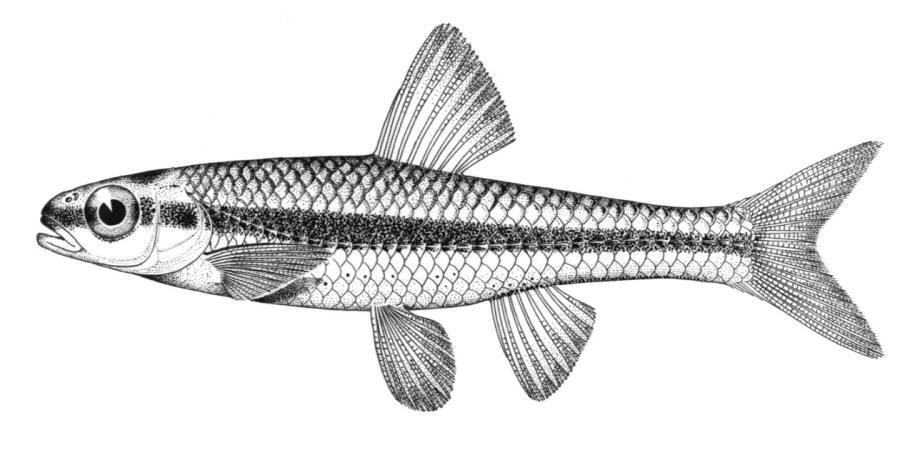
Taxonomy
Another common name is Black-striped Minnow.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is separated by having 6-7 branched anal fin rays, and a strong, black, zig-zag, mid-flank stripe extending onto the head including the upper lip and particularly the chin.
Description
Dorsal fin branched rays 6-7, usually 7, pectoral rays 12-14, and pelvic rays 7-8. Lateral line scales 31-38, the lateral line interrupted or incomplete in some fish. Short gill rakers number 6-8. Pharyngeal teeth hooked with a cutting edge, 1,4-4,1, 1,4-4,0, 1,3-4,1 or 4-4. Breeding males have minute nuptial tubercles on the top of the head and pectoral fin rays.
Colour
The back is yellowish, the flanks silvery and the belly white to yellowish. Upper flank scales are outlined with black but there is a gap or light stripe of unmarked scales between them and the flank stripe. Fins are transparent. Breeding males are a bright golden yellow. Peritoneum silvery to dusky.
Size
Reaches 7.1 cm standard length.
Found in the Great Lakes basin except northern Lake Superior, including southwestern Québec, southern Ontario, the Quetico region west of Lake Superior, and also southern Manitoba. Also in the upper Mississippi River basin.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Blackchin Shiners prefer clear, weedy water of pools in rivers and streams or lakes inshore. It schools over dense vegetation. It is intolerant of turbidity, silted bottoms and weed loss. Bottoms are bedrock, boulders, gravel or sand-mud mixtures. It may be limited by water temperature, preferring cooler waters.
Age and Growth
Life span is 4 years with maturity attained at 1 year.
Food
Food is planktonic crustaceans, small aquatic insects, terrestrial insects such as flies taken at the water surface, oligochaetes, and diatoms from bottom ooze. Food is also taken off vegetation. Peak feeding occurs at dawn and dusk.
Reproduction
Spawning occurs in May to July, and as late as August in Wisconsin. Fish from the NCR had partially developed tubercles on 29 June indicating spawning at a later date (McAllister and Coad, 1975). Eggs are up to 1.2 mm in diameter, adhesive on submerged plants and number up to 1800.
Importance
This species has been used as a bait fish and as a consequence of its ease of maintenance in aquaria, as a laboratory species. This species was placed in the "Not at Risk" category in 1994 by the Committee on the Status of Endangered Wildlife in Canada (Houston, 1996a).
Blacknose Shiner / Museau noir
Notropis heterolepis Eigenmann and Eigenmann, 1893
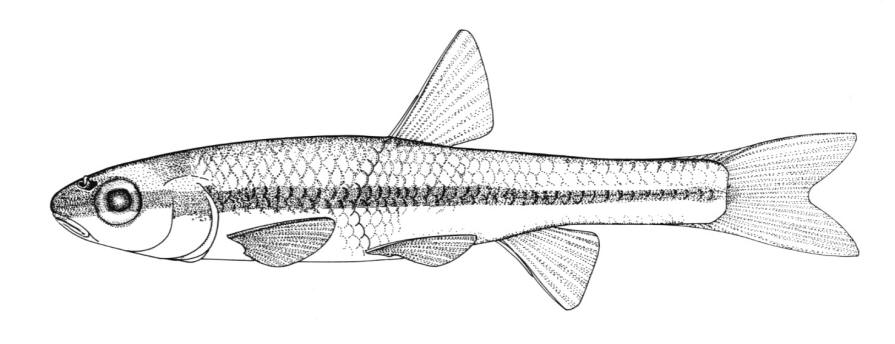
Taxonomy
Other common names include Northern Blacknose Shiner, Blacknose Dace, Black-sided Minnow, Muskoka Minnow, Cayuga Minnow and Blunt-nosed Minnow.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is separated from its relatives by having anal fin branched rays usually 7, often 6, 32-40 scales in a usually complete lateral line, a dark lateral stripe running onto the snout but not the chin (best seen in dead fish), and a dorsal fin origin over or behind the pelvic fin insertion level.
Description
Dorsal fin branched rays 6-8, pectoral rays 12-14 and pelvic rays 7-8. The lateral line may not be complete. Gill rakers are short and number 6-8. Pharyngeal teeth 4-4, slightly to strongly hooked at their tips. Breeding males have fine tubercles on top of the head and the first pectoral fin ray is thickened.
Colour
The back is yellowish to olive, flanks silvery and the belly silvery-white. Scales on the back are outlined with black pigment. The anterior flank stripe is made up of a series of crescent-shaped marks, the tips pointing rearward. The chin is white. Fins are mostly clear. Peritoneum silvery with some darker speckles.
Size
Reaches 9.5 cm.
Found from Nova Scotia and southern New Brunswick through southwestern Québec, Ontario, to southern Manitoba and Saskatchewan. In the U.S.A. southward in a narrowing distribution to Missouri and Tennessee.
Origin
This species entered the NCR from a Mississippian or possibly a Missourian refugium (Mandrak and Crossman, 1992).
Habitat
Blacknose Shiners are found in small, quiet streams, marshes and lakes which are clear and weedy. The bottom is usually sand, gravel or mud and detritus. They are intolerant of turbidity and are schooling fish over vegetation during the day. They can survive winterkill conditions unlike many other related minnows. Temperatures up to at least 28°C are tolerated in the NCR (McAllister and Coad, 1975).
Age and Growth
Life span is about 3 years with most growth occurring in the first year. Maturity is attained at 1 year.
Food
Food is aquatic insects both larval and adult, crustaceans, molluscs, sponges and algae, mostly taken from the bottom, peaking at dawn and dusk.
Reproduction
Spawning occurs in June and July in Ontario over sand bottoms. Fish in the NCR have been caught with large eggs on 13 June (McAllister and Coad, 1975). Yellow eggs may number up to 1420 per female with a diameter of 0.9 mm and are adhesive on the sand.
Importance
This shiner has been sold as bait in Ontario.
Spottail Shiner / Queue à tache noire
Notropis hudsonius (Clinton, 1824)
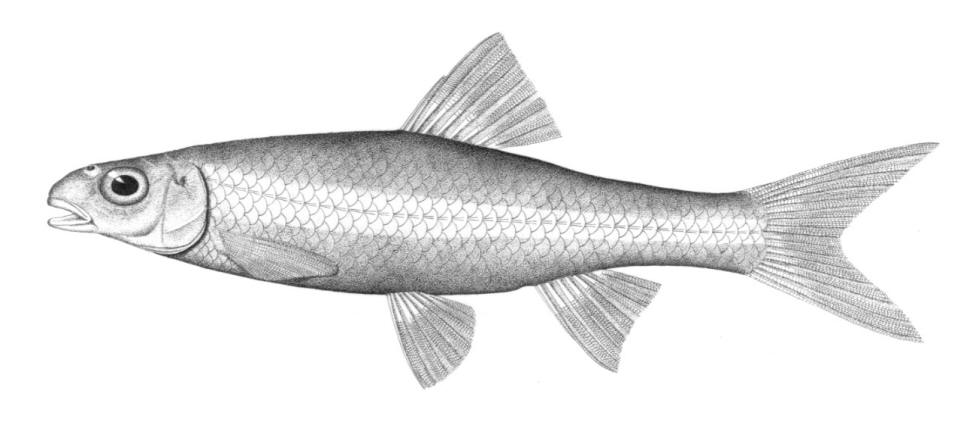
Taxonomy
Other common names include Spawneater and Sucking Carp.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is separated from its relatives by having 7 (rarely 6 or 8) anal fin branched rays, a subterminal mouth and a diffuse lateral stripe ending in a spot at the caudal fin base.
Description
Dorsal fin branched rays 7-8, pectoral rays 12-17 and pelvic rays 7-8. Lateral line scales 34-42. Short gill rakers number 7-11. Pharyngeal teeth 2,4-4,2 with a wide range of variations (even 4-4), hooked at the tip. Breeding males have tubercles on the top of the head, up to 10 lining scales on the back, occasionally on the branchiostegal rays, and on pectoral fin rays 7-10.
Colour
The back is yellowish, pale green or olive and the belly silvery-white. The tail spot may be covered by the silvery flank colour but is obvious in young and dead fish. Fins are transparent. The lower caudal fin rays are whitish. Peritoneum silvery with scattered dark melanophores.
Size
Reaches 14.7 cm total length.
Found from southwestern Québec, most of Ontario, Manitoba, Saskatchewan, Alberta, British Columbia and Great Slave Lake tributaries and the Mackenzie River system. In the U.S.A. south to Georgia in the east, but absent south of the Great Lakes, and to Iowa and Missouri in the west.
Origin
This species entered the NCR from a Mississippian or an Atlantic coastal refugium.
Habitat
Spottail Shiners are found in large lakes and rivers with slow to moderate current and clear water. The habitat bottom is sand, gravel, mud or silt and it has been caught as deep as 46 m. There is a movement into shallow water at night. In winter there is a retreat to deep water with individuals scattering. They can survive summer temperatures up to 35°C but their preferred temperature is a relatively cool 14.3°C. They occur in dense schools, usually in shallow water, with larger fish below smaller fish. The tail spot is a recognition mark enabling other school members to recognise conspecifics and position themselves appropriately in the school. The spot may also resemble an eye to predators which strike at the wrong end and miss as the Spottail darts away. A whole school of these eyes must be very confusing. The disappearance of these spots as the shiner attempts to escape will also trigger neighbouring members of the school to scatter.
Age and Growth
Life span is 5 years. Males grow more slowly than females. Some fish mature at age 1 and all are mature at age 3.
Food
Food includes plankton, aquatic and surface insects, molluscs, algae, and eggs and fry of their own and other species. It is a very important food for all predatory fish found with them in Canada.
Reproduction
Spawning occurs over sand in May to July in Canada, the timing depending on locality and its environmental conditions. In the NCR fish with large eggs at maximum size have been caught on 15 May (McAllister and Coad, 1975). It may be delayed until September in Wisconsin after a cold spring. In Iowa there are spawnings in May-June and in August. Yellow eggs number up to 8898 with a diameter of 1.1 mm. There are large spawning groups near stream mouths over gravel riffles or on sandy shoals. The eggs are adhesive.
Importance
This shiner has been used as a bait fish in Ontario and Québec, and as a biomonitor fish to determine the distribution through time and space of organochlorine contaminations (PCBs, mirex, dioxin and others) in the Niagara River, Lake Erie and Lake St. Clair.
Rosyface Shiner / Tête rose
Notropis rubellus (Agassiz, 1850)
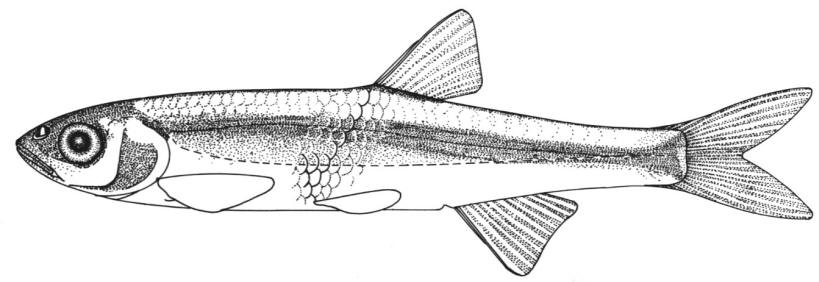
Taxonomy
Another common name is Skipjack.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is separated from its relatives by body depth being less than head length, dorsal fin origin is posterior to the pelvic fin insertion, pectoral fin rays usually 11-14, snout length is greater than eye diameter in adults, and flank pigment ends at or below the lateral line.
Description
Dorsal fin branched rays 6-7, anal branched rays 8-10, pectoral rays 11-14 and pelvic rays 8. Lateral line scales 36-45. The short gill rakers number 6-10. Pharyngeal teeth 2,4-4,2, with a wide range of variants. Breeding tubercles in males are found on the head, numbering over 100, in a row of about 12 on the lower jaw, on scales predorsally and above the lateral line, the latter edged with 1-5 tubercles, and on the upper pectoral and pelvic fin surfaces, and on the dorsal and anal fins. Females may develop tubercles on the head. Males have larger pectoral fin rays than females.
Colour
The back is olive to yellowish or bluish, often with a thin dusky stripe, flanks silvery with a thin, iridescent green or emerald or orange stripe above the lateral line, and belly is silvery-white. Scales are outlined with pigment. Fins are transparent. Breeding males are orange-red to brick-red on the head, intense blue on the back, a light red on the belly and pink on the dorsal, anal, pectoral and caudal fin bases. There is a scarlet bar at the gill opening. Females may develop a red colouration although this is paler than in males. Peritoneum silvery with dark speckles.
Size
Reaches 9.2 cm total length.
Found from southwestern Québec west to southeastern Lake Superior but absent from the rest of Lake Superior, throughout the southern Great Lakes and southern Ontario, and in the Red River of Manitoba. In the U.S.A. south to Virginia and northern Alabama.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
This shiner is found in clear streams and rivers over gravel and sand in fast rather than still water. It occurs less frequently in lakes. The bottom is usually bedrock or sand. It is a schooling species in the lower parts of streams where these meet larger streams or rivers. It does not tolerate turbidity or silt. It may be intolerant of the highest summer temperatures as its preferred temperature is 26.8°C.
Age and Growth
Life span is 3 years with maturity attained at 1 year.
Food
Food comprises aquatic and terrestrial insects, diatoms and algae, and some fish.
Reproduction
Spawning females contain up to 1482 eggs which are 1.5 mm in diameter. Fertilised eggs are said to turn bright yellow. Orange or pink-orange eggs are shed over gravel depressions near riffles when groups of 8-25 fish quiver and thrash for 5-6 seconds, breaking the water surface. The eggs are adhesive and fall in between the gravel where they and the larvae develop. Spawning in Ontario is from mid-May to late June at 20-27°C; 26 May to 28 June and 20.0-28.9°C in the Outaouais (Thellen, 1994). Spawning may occur over Hornyhead Chub and lamprey nests or with Common Shiners. Males engage in head butting and parallel swimming but do not defend a defined territory.
Importance
This species has been used as a bait fish in the U.S.A. and is a brilliant aquarium fish although difficult to keep. The eastern population of this species was placed in the "Not at Risk" category in 1994 by the Committee on the Status of Endangered Wildlife in Canada (Houston, 1996b).
Sand Shiner / Méné paille
Notropis stramineus (Cope, 1865)
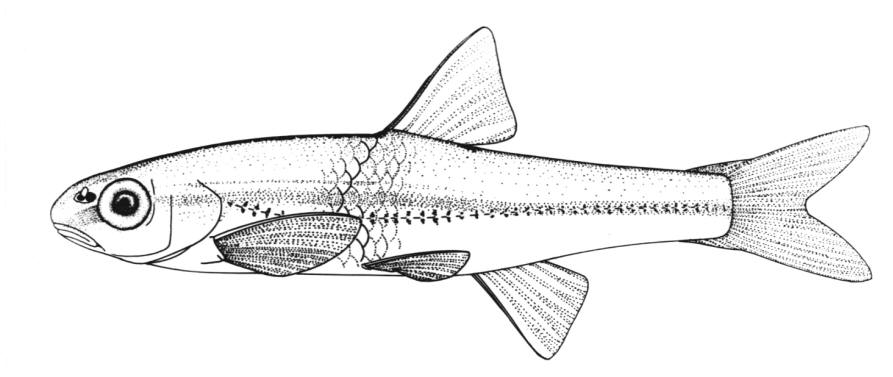
Taxonomy
Other common names include Shore Minnow and Straw-coloured Minnow. The scientific name Cyprinella ludibunda Girard, 1856 has priority and has been used in a limited fashion. The International Commission on Zooological Nomenclature ruled to conserve the commonly used Notropis stramineus (Anonymous, 2002).
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. It is identified by having usually 6 branched anal rays (range 5-7), a weakly developed mid-flank stripe best developed posteriorly not passing through the eye onto the snout, no black pigment concentrated about the anus or anal fin base, no pigment below the lateral line, and a thin stripe along the middle of the back before and behind the dorsal fin but not encircling it.
Description
The pupil has a wedge or "nipple" on its anterior margin that allows additional light to enter the eye for better forward vision. Dorsal fin branched rays 7, pectoral rays 12-18, and pelvic rays 7-8. Lateral line scales 31-39. Gill rakers are short and number 6-8. Pharyngeal teeth 4-4, hooked at the tip. Breeding males have minute tubercles on the head, cheeks and lower jaw and on pectoral rays 1-9 and on pelvic rays.
Colour
The back is light yellowish to olive-yellow, often almost transparent, flanks silvery with green and lavender tinges or a purplish iridescence, and belly silvery-white. Back and upper flank scales are outlined in black. There is a narrow, yellow stripe above the silvery flanks. Lateral line pores outlined with pigment, a spot above and a spot below each pore. Fins are colourless although anal fin membranes may be milky-white in breeding adults. Breeding adults develop a deeper yellow colour on the back. Peritoneum silvery with some darker speckles.
Size
Reaches 8.1 cm and 4.74 g.
Found from southwestern Québec, southern Ontario but not north of Lake Superior, southern Manitoba and southeastern Saskatchewan. In the U.S.A. south to Gulf drainages and to Mexico west of the Appalachian Mountains. It is also recorded from a tributary of Wolf Creek near the Larose Forest in the South Nation River basin (not on map here) (Van Dusen, 2011).
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
As the name suggests, this shiner prefers sandy areas of lakes and large rivers, often with little current. It may descend to 34 m. It may also be found in pools below riffles in smaller streams. The water is usually clear and plants are few. There are daily movements of this schooling fish from deep water in the day to shallows at night.
Age and Growth
Life span is at least 3 years with maturity at 1 year.
Food
Food includes aquatic insects, zooplankton, algae, bottom crustaceans, diatoms and terrestrial insects taken at the surface.
Reproduction
Spawning occurs from April to August depending on latitude and water temperature regime. Temperatures have been reported to be as high as 37°C. July appears to be the spawning peak in Michigan and Wisconsin. A female can contain up to 2660 eggs of 1.0 mm diameter. Eggs are shed over gravel or sand, perhaps under submerged vegetation, and are adhesive.
Importance
None.
Mimic Shiner / Méné pâle
Notropis volucellus (Cope, 1865)
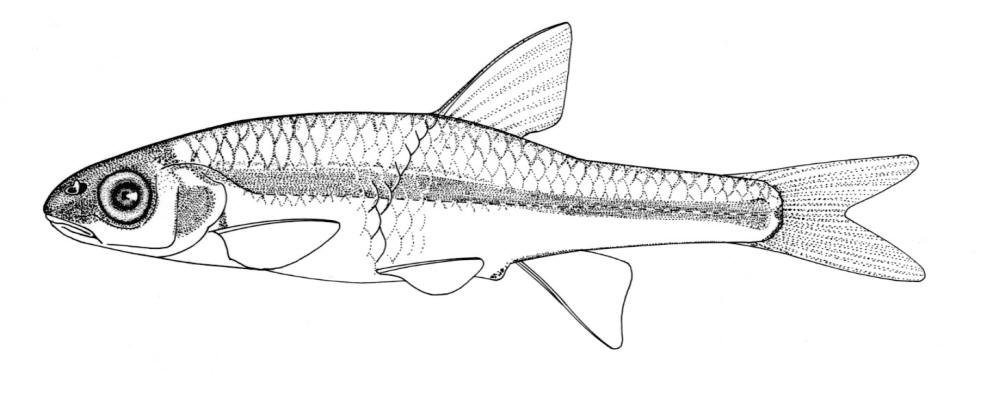
Taxonomy
Another common name is Channel Mimic Shiner.
Key Characters
This species and other shiners (genera Cyprinella, Luxilus and Notropis) are separated from other family members by usually having 7 branched dorsal fin rays following thin unbranched rays, protractile premaxillaries (upper lip separated from the snout by a groove), no barbels, large lateral line scales (fewer than 50), and a simple, s-shaped gut. This species is separated from its relatives by having 7 (rarely 8) anal fin branched rays, a dusky lateral band on the flank, black pigment around the anus and anal fin base, no clearly defined mid-dorsal stripe and pigment extends below the lateral line.
Description
Dorsal fin branched rays 7, pectoral rays 12-16 and pelvic rays 8-10. Lateral line scales 32-40 with the anterior scales twice as high as wide. Pharyngeal teeth 4-4, slightly to strongly hooked. Breeding males have minute tubercles on top of the head, snout and to a lesser extent on the preopercle and lower head, and on the pectoral rays. Upper pectoral rays are thickened.
Colour
Back with dark outlined scales on a straw-yellow to yellowish-olive background. Flanks are silvery with the dark lateral stripe poorly developed anterior to the dorsal fin level but sometimes ending at the caudal base in a triangular spot. Lateral line scales have a spot above and below each pore. The belly is white. Peritoneum silvery with some darker speckles, or dusky.
Size
Reaches 7.7 cm.
Found from southwestern Québec through southern Ontario and the Great Lakes to northwestern Ontario and southern Manitoba. South to Mexico and Alabama.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Mimic Shiners prefer still water in medium-sized streams and lakes and school at mid to surface levels. They may also be found in fast current of streams and rivers, at the base of rapids or falls. The bottom can be sand and gravel with some silt or bedrock and are often found in water less than 1 m deep. Vegetation may be present. During the night, Mimic Shiners remain on the lake bottom scattered on bare areas. They aggregate into schools of up to 20,000 fish during the day.
Age and Growth
Life span is rarely 3 years and maturity is attained at 1 year.
Food
Food is crustaceans, aquatic and terrestrial insects, and algae and detritus browsed from the bottom. Most feeding occurs at dawn and dusk. Predators include Smallmouth and Largemouth Bass, other fishes and various birds.
Reproduction
Spawning occurs from May to August in Wisconsin and orange eggs are thought to be broadcast over vegetation at night, up to 960 per female. Thellen (1994) gives a period of 20 June to 9 July for the Outaouais. Egg diameter is 1.0 mm. The gonadosomatic index of this fish in Manitoba peaked at 20°C for females and 15°C for males. Spawning there was over a three-week period in July.
Importance
None.
Northern Redbelly Dace / Ventre rouge du nord
Phoxinus eos (Cope, 1862)
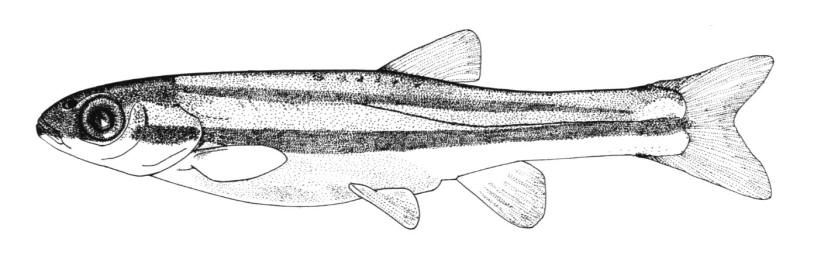

Taxonomy
Another common name is Red-bellied Dace. Formerly in the genus Chrosomus, this species is now placed with its Eurasian relatives It hybridises freely with the related Finescale Dace, with the hybrids interbreeding, making some populations a mixture of intermediate forms, none clearly one dace or the other. Such hybrids are known from Hawley Lake, Gatineau Park. As well as diploid hybrids, mosaic and triploid hybrids have been found. Hybrids may be produced continually by the parental species or be a distinct, self-perpetuating lineage. Research has shown them to be a unisexual, clonal hybrid species perpetuated by gynogenesis, i.e. the females produce eggs identical genetically to themselves but require sperm from a related species (often one of the parental species) to stimulate development. The male makes no genetic contribution. This explanation of the all-female species is borne out by some populations now having only one of the parental species present so hybrids can no longer be produced (Joswiak et al., 1985; Dawley et al., 1987; Dawley and Goddard, 1988; Goddard et al., 1989).
Key Characters
This species has protractile premaxillaries (a groove between the upper lip and the snout), no barbels, an incomplete lateral line and minute scales in lateral series 66-95, a black peritoneum and, in contrast to its relative the Finescale Dace, mouth not extending past the anterior eye margin, 2 extra major loops in the long gut and a stripe or series of spots between a dark upper flank stripe and the back.
Description
Dorsal fin branched rays 6-8, anal branched rays 6-8, pectoral rays 13-16 and pelvic rays 8-9. Scales have radii on all fields. The lateral line usually does not pass the level of the pelvic fin origin. Pharyngeal teeth 5-5 with variants 5-3 and 5-4. Males have tubercles lining breast scales to form 4-6 comb-like rows. Minute tubercles are found on the head, above the anal fin, lower half of the caudal peduncle and pectoral fin rays. Larvae from Ottawa are described by Faber (1985).
Colour
The back is olive, dark brown or even black, lower flank silvery to cream below a broad, dark, mid-flank stripe. The upper flank stripe breaks up into spots behind the dorsal fin. Between the two flank stripes the body is silvery-yellow and there is a thin oblique line trending posteriorly, below the dorsal fin. There is a stripe on the middle of the back. Breeding males have a brilliant red lower flank and belly below the mid-flank stripe. The lower flank is yellow before and after the peak spawning period. Females are yellowish-green below the mid-flank stripe in the spawning season. Fins are yellowish. Larval pigment patterns are described by Faber (1984b; 1985b).
Size
Reaches 7.7 cm.
Found from P.E.I. and Nova Scotia west through New Brunswick, southwestern Québec, probably all of Ontario but only southern Manitoba, and southwestern Saskatchewan, much of Alberta except eastern parts, the eastern Peace River basin of British Columbia and in the N.W.T. only south of Great Slave Lake. In the U.S.A. south to Pennsylvania and Colorado.
Origin
This species may have entered the NCR from Mississippian or an Atlantic coastal refugium (McAllister and Coad, 1975) or from a Mississippian or possibly Missourian refugium (Mandrak and Crossman, 1992).
Habitat
The Northern Redbelly Dace inhabits the brown waters of bogs, ponds and small, slow-moving streams over silt or detritus bottoms, sometimes over sand or gravel. Its preferred temperature is 25.3°C. In lakes, this fish is found in schools which break up into individuals for feeding at sunset. At this time they are subject to predation but injured fish release an alarm substance. This induces other individuals to move closer to the substrate, away from the injured fish, show more dashing and freezing movements, and form more cohesive schools. The alarm substance even allows fish to assess the risk of being eaten by a predator, by the concentration of the substance in the water. This species and its hybrid with P. neogaeus are infested with black spot disease in Lake Fortune, Gatineau Park (Paradis and Chapleau, 1994). The black spots are present on 87.3% of captured fish, are most prevalent on the fins, and the number increases with age up to as many 216. However the infestation does not appear to affect the biology of this fish.
Age and Growth
Life span is 8 years for females and 6 years for males. Maturity may be attained as early as 1 year of age.
Food
Food is diatoms, filamentous algae, zooplankton and aquatic insects. Various fish-eating birds and Brook Trout eat this species.
Reproduction
In southern Canada spawning occurs in late May to August. Fish in the NCR have been caught containing large eggs in May (McAllister and Coad, 1975) and larvae in June (Faber, 1985b). Some populations in central Ontario have an extended spawning season, from mid-June to mid-August. These fish were fractional spawners, releasing eggs in batches over a long period. Temperatures are usually above 13°C. A female, with 1-8 males in attendance, darts in and out of filamentous algae, depositing non-adhesive (other reports state that eggs are adhesive) eggs in a struggling mass. About 5-30 eggs are shed at each dart into the algae. A female can contain up to 6450 eggs of 1.24 mm diameter. Eggs take 8-10 days to hatch at 21.1-26.6°C and probably larvae attach to plants and other objects with cement glands on their heads. Larvae are 5.0-6.0 mm long at hatching. Development from larvae to juveniles takes 25-30 days (Faber, 1985b).
Importance
This dace is used as a bait fish in eastern Canada, for such species as Walleye.
Finescale Dace / Ventre citron
Phoxinus neogaeus Cope, 1867
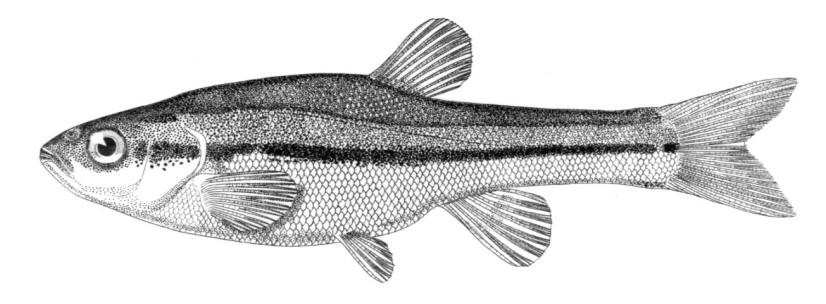
Taxonomy
Other common names include Bronze Minnow, New World Minnow, Rainbow Chub and Leatherback. Formerly in the genus Chrosomus. This species hybridises readily with the Northern Redbelly Dace (q.v.).
Key Characters
This species has protractile premaxillaries (a groove between the upper lip and the snout), no barbels, an incomplete lateral line with scales in lateral series 63-92, a black peritoneum and, in contrast to the Northern Redbelly Dace, a mouth extending to below the anterior eye margin, a short, s-shaped gut, and no upper flank stripe.
Description
Dorsal fin branched rays 6-8, usually 7, anal branched rays 6-8, usually 7, pectoral rays 12-16 and pelvic rays 7-8. Pharyngeal teeth hooked and usually 2,5-4,2 but also 2,5-5,2, 2,5-4,1 or 1,5-4,1. The lateral line usually ends above the pelvic fin origin and has 9-34 pores. Scales are small and have radii on all fields. Spawning males have 4-5 scale rows on the breast edged with tubercles in comb-like rows and 4-5 rows of tubercles on scales above the anal fin base and on the lower caudal peduncle. The first 4-5 pectoral rays are thickened and darkened and membranes are swollen in breeding males. The first ray has a distinct notch about one-quarter of the fin length from the base.
Colour
The back and upper flank are brown to black, below is an olive-green to gold stripe and then a dark mid-flank stripe which extends onto the head and lips but not the chin, and ends in a spot at the caudal fin base. A thin dark line extends obliquely from the dark back to the flank stripe. The body has numerous, small, black speckles. Breeding males are bright chrome yellow to red below the dark mid-flank stripe. The lower flank is silvery-white to cream. Fin rays are outlined by dark pigment, most intensely during the breeding season.
Size
Reaches 10.7 cm.
Found from Maine, New Brunswick and southwestern Québec throughout the Great Lakes and adjacent U.S. states and all of Ontario, in southern Manitoba, in most of Alberta, the southwestern corner of Saskatchewan, northeastern British Columbia and down the Mackenzie River valley. Also in Wyoming, Colorado, the Dakotas and Nebraska.
Origin
This species entered the NCR from a Mississippian or possibly a Missourian refugium (Mandrak and Crossman, 1992).
Habitat
This schooling dace favours bogs, ponds, lakes and slow-moving streams with mud or mud and gravel bottoms. The water is clear or tea-coloured.
Age and Growth
Life span is 8 years with both sexes mature at 2 years.
Food
Food is aquatic insects, crustaceans, molluscs and plankton. Terrestrial insects taken at the water surface, such as ants, as well as spiders can be eaten.
Reproduction
Spawning occurs in April-July at 11°C water temperatures or higher. Spawning take space over a shorter period than for the Redbelly Dace. Both males and females initiate chases, with males nipping at the anal and caudal region of a female. The females initiate chases by swimming in a zig-zag fashion. A male swims alongside a female and places his enlarged pectoral fin under the anterior part of the female's body, so that he can direct her swimming to some degree. The male tries to press the female against a rock or perhaps in holes under logs or branches, curling his tail over her tail so that his tubercles above the anal fin are close to her vent. The pair vibrate for several seconds and sperm and about 20-40 eggs are shed. A female can have up to 3060 eggs of 1.5 mm diameter when water hardened.
Importance
Finescale Dace have been used as bait fish in Québec and Ontario, for such species as Walleye.
Bluntnose Minnow / Ventre-pourri
Pimephales notatus (Rafinesque, 1820)
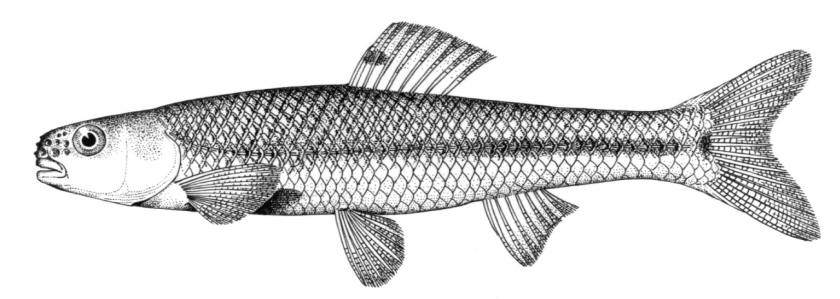

Taxonomy
Other common names include Bluenosed Chub, Bullhead Minnow and Fat-head Chub.
Key Characters
This species is identified by having premaxillaries protractile (lip separated from the snout by a groove), no barbel, and uniquely in this species and the Fathead Minnow the first, unbranched dorsal fin ray is short and separated by a membrane from the next, longer ray. It is separated from the Fathead Minnow by the scales in front of the dorsal fin being much smaller and more crowded than flank scales, the back is flattened, the lateral line is complete, the mouth is under the snout and there is a caudal fin base spot.
Description
Dorsal fin branched rays 7, anal branched rays 6-7, mostly 6, pectoral rays 14-17 and pelvic rays 8. Lateral line scales 37-50. Gill rakers are short and number 8-10. Pharyngeal teeth are 4-4, hooked and serrated, and the gut is elongated with several coils. Males have 3 rows of 14-17 large tubercles over the snout anterior to the nostrils and small tubercles on the upper pectoral fin rays. Breeding males have a fleshy papilla at each mouth corner, rather like a barbel.
Colour
The back is olive-green to sandy-brown, the flanks silvery and the belly white. There is a dusky flank stripe from snout to tail. Scales are outlined in black. Fins are yellowish and the dorsal fin has a dusky blotch just above the anterior base. Breeding males become almost black to bluish-black overall with a whitish spongy pad on the anterior back. Peritoneum black. Faber (1984c) illustrates a larva.
Size
Reaches 11.2 cm.
Found in southwestern Québec, southern Ontario but not north of Lake Superior, Lake of the Woods and southern Manitoba. In the U.S.A. south to Virginia in the east and in the Mississippi River basin.
Origin
This species entered the NCR from possibly a Mississippian or an Atlantic coastal refugium.
Habitat
Bluntnose Minnows are found in lakes, ponds, marshes and streams over sand, gravel, mud or silt. The area may be heavily vegetated and the current still to medium. Their preferred temperature is 29.0°C.
Age and Growth
Life span is at least 3 years for males. Males grow much larger than females. Females mature at 1 year and males at 2 years of age.
Food
Food is detritus and includes organic matter, chironomid and other insect larvae, diatoms and algae. Algae may be taken particularly in winter. Some surface insects, plankton and worms are also taken as well as its own eggs and fry and those of other fish species. Various other fishes are predators of this species.
Reproduction
Spawning occurs in late May to August at 19°C water temperatures or warmer. Eggs are laid at intervals through the season, 5-547 at a time, in shallow water (under a metre deep). Clutches laid varied between 7 to 26 per female. A spawning season at any one locality was about 7 weeks in one study to 3 months in another. Each female has up to 10,164 eggs in her ovaries in several stages of development, up to 1.5 mm in diameter. This level of egg production exceeds that of other species like the "prolific" Common Carp in terms of body weight. Generally those fish species that build nests and guard eggs are supposed to produce fewer eggs which have a better chance of individual survival than the numerous eggs produced and abandoned by prolific species. The Bluntnose and Fathead Minnows are exceptions to this general rule. The male defends a nest in shallow waters. The nest is a space under a stone, log, clam shell or even a board or tin can, cleaned out with the aid of his tubercles and tail sweeps. The undersurface of the stone is cleaned with his mouth and spongy back pad and eggs are deposited there mostly at night but also during the day. The male and female turn on their sides under the nest stone with the male under the female, pressing against her. The male rapidly arches his caudal peduncle against the female's urogenital region, the female rapidly undulates through an s-shape and an egg is extruded. The egg is transferred to the upper side of the female and the undulation rolls it along between her side and the nest cavity roof. When the adhesive egg reaches the tail she presses it against the roof where it sticks. How the female transfers the egg from her papilla to her side is unknown and the question remains why this sideways process is used when other roof spawners simply turn upside down and deposit eggs directly. The process may serve to roll eggs into a vacant roof space, occupying the roof most efficiently. Eggs laid on top of others may not attach well or prevent proper development of the underlying eggs. Nest construction takes about 1-2 hours. Over 5000 eggs at various developmental stages from several females are found in a single nest. The male guards the eggs, removes dead ones, and keeps them free of silt and aerated until they hatch. Larvae are 5.0-6.0 mm long at hatching.
Importance
Bluntnose Minnows are used as bait fish in Canada, and in the U.S.A. where they have been raised in ponds for distribution as a forage fish for game species. This fish was placed in the "Not at Risk" category in 1998 by the Committee on the Status of Endangered Wildlife in Canada.
Fathead Minnow / Tête-de-boule
Pimephales promelas Rafinesque, 1820
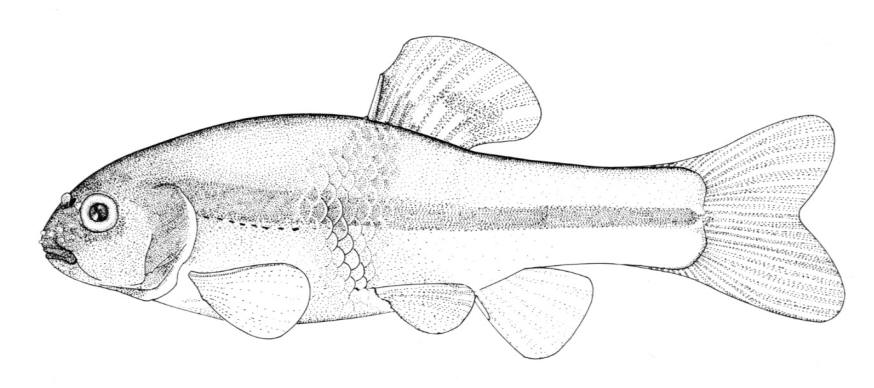

Taxonomy
Other common names include Northern Fathead Minnow, Blackhead Minnow and Tuffy Minnow.
Key Characters
This species is identified by having premaxillaries protractile (lip separated from the snout by a groove), no barbel, and uniquely in the this species and the Bluntnose Minnow the first, unbranched dorsal fin ray is short and separated by a membrane from the next, longer ray. It is separated from the Bluntnose Minnow by having scales in front of the dorsal fin much smaller and more crowded than flank scales, the back is flattened, the lateral line is incomplete to nearly complete, the mouth is at the tip of the body and a caudal fin base spot is faint to absent.
Description
Dorsal fin branched rays 7, rarely 8, anal branched rays 6, sometimes 7, pectoral rays 14-18 and pelvic rays 8-9. The short gill rakers number 13-16. Pharyngeal teeth 4-4, slightly hooked at the tip with elongate cutting surfaces. The gut is elongate with several loops. Scales in lateral series 39-56, lateral line pores usually ending before the dorsal fin origin. Large tubercles in males are found on the snout in 3 rows with 4-15 in the lower row, and with up to 11 tubercles on the lower jaws. There are also smaller tubercles on top of the head and as single rows on the pectoral rays. Males also develop a blue-black to grey, spongy, wrinkled pad on the back between the head and dorsal fin. Fin membranes swell. Females lack the pad and tubercles but their vent region is swollen and there is some swelling of fin membranes, particularly of the anal and pelvic fins near the vent.
Colour
The back and upper flank are dark olive-green to brown, silvery to golden flanks and a whitish belly. There is a mid-flank stripe. Breeding males darken, particularly the head and dorsal fin, and the stripe is not apparent. The body can be completely black with a white band at the head-body region and under the dorsal fin. This occurs only during aggression or sexual activity. The lateral banding enhances the robust appearance of fish which must maintain a territory over several weeks without much opportunity to feed. Weight loss is replaced by water to help maintain the image of a fat and vigorous male. Very frightened fishes blanch. Females are quite dowdy and lack the darker colouration of males. Peritoneum black. The Rosy-red Minnow with orange-red body and fins is an aquarium variety. Faber (1984c) illustrates a larva.
Size
Reaches 10.2 cm.
Found from western New Brunswick through southwestern Québec, Ontario, southern and central Manitoba and Saskatchewan, Alberta, British Columbia (introduced) and southern tributaries of Great Slave Lake, N.W.T. South in central regions of North America to Mexico.
Origin
This species entered the NCR from a Mississippian or possibly a Missourian refugium (Mandrak and Crossman, 1992). The presence of this species in Gatineau Park may be from introductions (Rubec, 1975a).
Habitat
Fathead Minnows are found in ponds, small to large lakes, and slow-flowing brooks to large and turbid rivers. It is often found in ditches and other artificial habitats because of its tolerance of poor conditions. The bottom is mud, detritus or sand and little current. They tolerate turbidity, high temperatures, pH variations, salinity and low oxygen. Their preferred temperature is 29.0°C. Fatheads sharing a lake with pike show sheltering, dashing and freezing behaviours more often than fatheads from lakes without pike. They also show stronger fright responses to water which pike have lived in - a chemical response.
Age and Growth
Males grow faster and larger than females, typical of nest defending species. Life span is about 4 years with maturity attained as early as 1 year, rarely in the year of birth.
Food
Food is bottom sediment for its organic content including plant material, aquatic insects and zooplankton. It is an important food for many other fishes and aquatic birds.
Reproduction
Fathead males in the NCR have been caught with developing tubercles as early as 13 April and tubercles are fully developed through late May to June and into July or even early August. In late July and early August males begin to lose their tubercles and by early September only scars remain (Coad, 1987a). Spawning runs from April to August, once water temperatures reach 14°C and light-dark hours are 16-8. Males choose a spawning site under a log, rock, plant stems or even a lily pad or any solid artificial structure such as a plank in shallow water. Cavity spawning in mud-bottomed habitats prevents the eggs from being smothered as well as offering the relatively few eggs spawned from a small fish protection from predators. The male will clean out the cavity, spending up to 10 hours on the task, using his tubercles to scrape, pulling debris with his mouth and sweeping with his tail fin. The spongy pad on the back may serve to test spawning sites and eggs chemically. The pad secretes a mucus which is smeared on the spawning site perhaps to improve it for egg survival since mucus protects against disease and parasites. Diseased eggs are eaten by the male. The mucus may also serve to indicate ownership of a nest site. Spawning male fatheads lose their ability to produce alarm or fright chemicals on skin injury, otherwise the continual pad rubbing would disturb spawning activities by releasing alarm substance and scaring away females. Females may enter the spawning site casually, be chased there by a male or enticed by a face to face encounter and leading to the nest. He lifts and presses the female on her side between himself and the roof of the spawning site. Egg deposition is thought to use the same unusual mechanism as in the Bluntnose Minnow (q.v.). Males court females by swimming rapidly up to them and then freezing at 3-5 cm away, and by leading females with a zig-zag or straight-line motion from the female to the nest site. Males will also display to females by erecting their fins for 2-3 seconds and by jump-swims in which a male swims upwards to a female then rolls on his side and swims back down. Butting and lateral quivering also occur, perhaps attempts to assess spawning condition of the female. Males defend the eggs against other fishes including female fatheads using the snout tubercles to butt and tail swipes to intimidate by sending a pressure wave sensed by the lateral line system. Chasing and biting are common and 2 males may carousel (or circle head-to-tail) trying to contact each other. Leeches and turtles are also driven away. Some eggs are lost while the male is distracted chasing away predators. Repeat spawning may be necessary to replace lost eggs. Males also aerate and clean the eggs with fin movements. A nest will contain eggs in various stages of development as the male will spawn with several females. Females will deposit eggs in several nests. Orange, mature eggs are up to 1.6 mm in diameter with 12,000 or more per nest. A female will release up to 10,164 eggs in a season but from 9 to 1136 at a time. Spawning intervals are 2 to 16 days. Larvae are 4.0-5.0 mm long at hatching.
Importance
In the U.S.A. fatheads are raised as bait fish and as forage fish for introduction into bass fishing lakes. They are also used extensively as a laboratory animal for tests of toxic compounds. They have even been used to evaluate the biological effects of materials from the moon.
Longnose Dace / Naseux de rapides
Rhinichthys cataractae (Valenciennes, 1842)
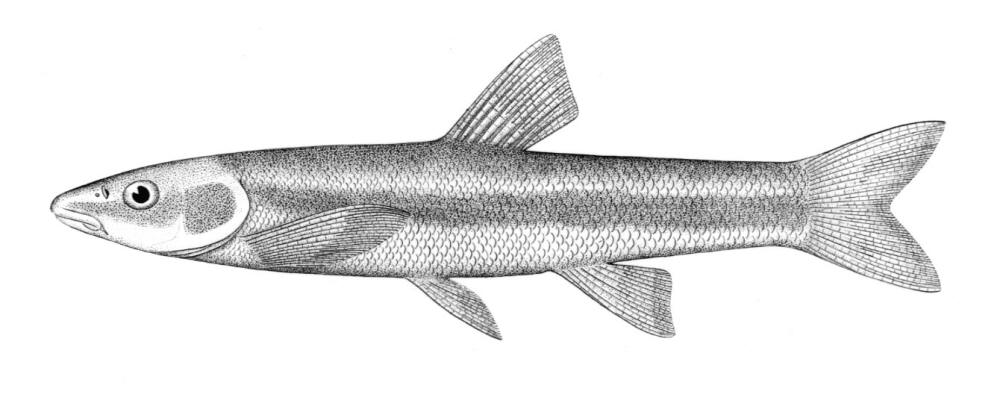
Taxonomy
Other common names include Great Lakes Longnose Dace and Stream Shooter. This species was described from fish collected at Niagara Falls, hence "cataractae".
Key Characters
This species is distinguished by the premaxillaries not being protractile (no groove between the upper lip and snout), a barbel at each mouth corner and an elongate snout projecting beyond the ventral and u-shaped mouth.
Description
Dorsal fin branched rays 7, anal fin branched rays 6, pectoral rays 12-17 and pelvic rays 7-9. The pectoral fins are large, low on the body and splayed. They are larger in males than females, reaching back to the pelvic fins. Lateral line scales 55-76. There are 6-8 short gill rakers. Pharyngeal teeth hooked, 2,4-4,2, 1,4-4,1 or 1,4-4,0. Breeding males have nuptial tubercles on the top of the head, posterior scale edges, and on the pectoral, pelvic, anal and dorsal fins. These tubercles are found from mid-May to early September and are best developed earlier in the year.
Colour
The back is olive-green to brown, grey or black fading to a cream or whitish belly. The lower flank and belly may be golden. Some scales may be darkly pigmented and there may be a dark stripe in front of the eye. The lateral line is wholly or partially dark, or not dissimilar from the background, varying with locality. Overall body colour can vary from almost black to silvery. The origin of the dorsal fin and the upper origin of the caudal fin are white to cream. Fin rays are outlined by dark pigment. Young fish have a dark stripe on the head and body. Breeding males have orange-red mouth corners, cheeks, pectoral fin axils and on the pelvic fins and anterior anal fin base. Dorsal and caudal fin membranes dull to dark red. The back and upper flank are dark olive. Peritoneum silvery with brown spots.
Size
Reaches 17.8 cm.
Found from Labrador and the upper St. Lawrence River basin north to Hudson Bay, west through the Great Lakes, to British Columbia and north in the Mackenzie River basin. Absent from northern Manitoba, Saskatchewan and northwestern Alberta and to the north. In the south it reaches Virginia east of the Appalachians, Iowa to the west and in a long western arm extends into Mexico.
Origin
This species entered the NCR from a Mississippian, Missourian or an Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
Longnose Dace are found in fast, clear streams with gravel, rock, and boulder beds, and in lakes with wave action. Their preferred temperature is 20.6°C but a wide range of temperature and turbidity is tolerated. It can live in very fast water by taking advantage of holes and crevices between rocks. The swimbladder does not grow with the fish so large specimens have relatively small swimbladders, are less buoyant and are able to hug the bottom. Fish which develop in still water have larger swimbladders than those in fast water. This affects the buoyancy range that can be adjusted in response to varying current speeds. Lake dwelling fish adjust their swimbladder volume in response to wave action. During the day this dace remains under stones, hiding from predators.
Age and Growth
Life span is 5 years with sexual maturity attained at 2 years, rarely at 1 year.
Food
Food is principally aquatic insects such as blackflies, caddisflies, midges and mayflies taken at night, an unusual feeding time for a Carp Family member. Some worms, terrestrial insects, crustaceans, molluscs and fish eggs are also eaten. Algae and diatoms may predominate in some populations during the summer. Smallmouth Bass and Brook Trout are predators of this dace, as are mudpuppies under the ice in Kemptville Creek (email from F. W. Schueler, 8 March 2003).
Reproduction
Spawning occurs from April to August at temperatures over 11°C over riffles, and sometimes over nests of other species. Females in the NCR have been caught on 15 May with eggs 1.5 mm in diameter at a water temperature of 17°C, close to spawning (McAllister and Coad, 1975). Males defend a territory up to 20 cm across and butt or bite intruders. When a female enters a male's territory he vibrates rapidly for 0.5-2.0 seconds at 0.75-1.0 second intervals and pushes his snout against rocks with his body sloping at 45-90°. The female responds by adopting the same angle. The two fish come together and push against the bottom, quiver for 1-2 seconds and release eggs and sperm. Eggs are adhesive, transparent, 1.7 mm in diameter and number up to 9953 per female. Young are pelagic and live near shore in slow or still waters for about 4 months before settling to the bottom.
Importance
Used as a bait fish in the U.S.A. for bass and catfish but not in Canada to any marked extent.
Creek Chub / Mulet à cornes
Semotilus atromaculatus (Mitchill, 1818)
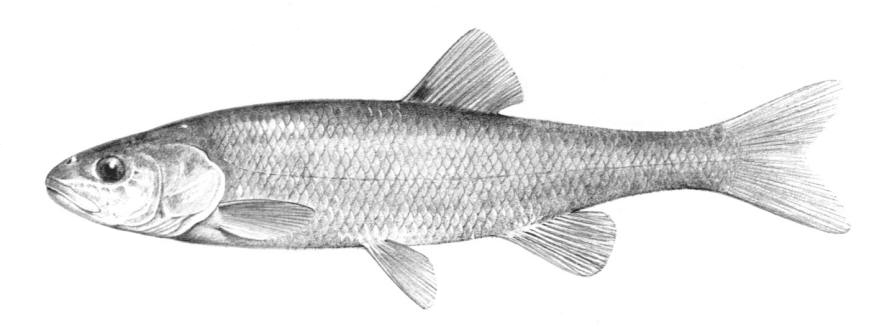


Taxonomy
Other common names include Horned Dace, Common Chub, Brook Chub, Tommycod, Silvery Chub, Mud Chub and Blackspot Chub. Hybrids are formed with Common Shiners and Longnose Dace as well as other species not in the NCR.
Key Characters
This species is distinguished by having protractile premaxillae (a groove between the upper lip and the snout), a flat barbel in advance of the mouth corner in the groove above the upper lip, lateral line scales 47-66 (usually 52-63) and a black spot at the anterior dorsal fin base.
Description
The upper jaw extends back to a level with the front of the eye so the mouth is large. Short gill rakers number 9-11. Pharyngeal teeth 2,5-4,2, 2,4-5,2, 2,5-5,2 or 2,4-4,2 with a strongly hooked tip. Dorsal fin branched rays 7-8, usually 7, anal fin branched rays 6-8, pectoral rays 13-20 and pelvic rays 7-9. Breeding males have up to 12 very large tubercles in a line on each side of the head from in front of the lip to over the eye. Smaller tubercles are present on the sides of the head and in a single row on up to 8 pectoral fin rays. Tubercles may also be present on other fin rays. Posterior flank scales have tubercles lining their margins and tubercles extend along the upper, anterior caudal fin lobe margin.
Colour
The back is olive, sometimes with a steel-blue tinge, the flanks silvery and the belly silvery-white to white. Flanks have iridescent purplish or greenish tinges. Scales are outlined with pigment. Fin rays are edged with pigment. The dorsal and caudal fins are dusky. Young are more silvery but still retain an iridescent purplish tinge, have a flank stripe ending in a spot at the tail base, and a reddish tail fin (although not as bright red as small Hornyhead Chubs). Breeding males may have rosy, orange or yellow tints on the head, body and dorsal fin base. Lower fins are orange and the lateral head surface is blue. Peritoneum silvery with some speckling.
Size
Reaches 33.0 cm.
Found from Nova Scotia westward through southern Québec and Ontario, including the Great Lakes and upper James Bay drainages, to southern Manitoba. Sporadic records in northern Québec. South to Gulf coast drainages and west to Montana in the U.S.A.
Origin
This species entered the NCR from a Mississippian, Missourian or an Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
The Creek Chub is very common in clear, smaller streams in pool areas but may also be found along lake shores. The bottom is usually boulders or gravel but may be mud-detritus.
Age and Growth
Life span is about 8 years with females maturing at age 2 and males at age 3. Males grew more rapidly than females in a study near Peterborough, Ontario.
Food
Food varies from plankton when young to aquatic and terrestrial insects, crayfish, frogs and small fishes, such as Johnny Darter and Brook Stickleback, and even berries as they grow larger. Some algae and higher plants may also be eaten. In a Québec lake, young fed on small adult flies and aquatic beetles during the day while adults were nocturnal and fed on larger prey, crustaceans and insect larvae. More benthic prey are available at night. Creek Chub are eaten by a wide variety of fish and birds.
Reproduction
Spawning occurs in April-July at about 12°C or higher near stream riffles. Their preferred temperature is 20.8°C. Males from the NCR caught on 15 May had tubercles but by 31 July only scars remained, indicating that the breeding season was over (McAllister and Coad, 1975). In the Mink River, Manitoba and near Peterborough, Ontario, most spawning occurs between the middle and the end of May. Males excavate a pit by violent body movements which dislodge smaller particles and by picking stones up in the mouth and dropping them upstream. This creates a pit which is continually filled in by the current covering and protecting the eggs. The pit is about 30 cm wide but can be more than 5 m long. Several males may collaborate on a single nest. A female approaches a male defending his nest and is tossed into an upright position by the male inserting his head and pectoral fin under her body. The male instantly embraces her in a horizontal curve in about a tenth of a second and about 50 eggs are shed into the gravel and fertilised. Tubercles help the male to grip the female. After this embrace, the female turns belly up as though stunned but soon swims off to spawn again in the same or other nests. A female continues spawning over several days until all eggs are shed. The male covers the eggs with gravel. A male may not lift a female up but instead press her to the bottom or against the pit side when eggs are released. Females have up to 7539 eggs of 1.7 mm diameter. Some males are "nest-watchers" who may spawn with a female while the dominant male is chasing away another rival. Fights appear to use the head tubercles as weapons although injuries have not been observed. Usually contending males parallel swim for several metres upstream from the nest, with fins erect, mouths open and tails beating slowly but strongly. Tail beats are meant to intimidate. Male Common Shiners often crowd around a Creek Chub nest involved in their own reproductive behaviour. They may even bump into the creek chub who will use his massive, tuberculate head to drive them away momentarily. Shiners and other fishes however can enter nest sites without eliciting a response from the chub unless he is spawning. All intrusions by other male chub result in aggression.
Importance
Creek Chub are used extensively in Canada as a bait fish and can be caught on a baited hook. They are good eating except for the nuisance of small bones.
Fallfish / Ouitouche
Semotilus corporalis (Mitchill, 1817)
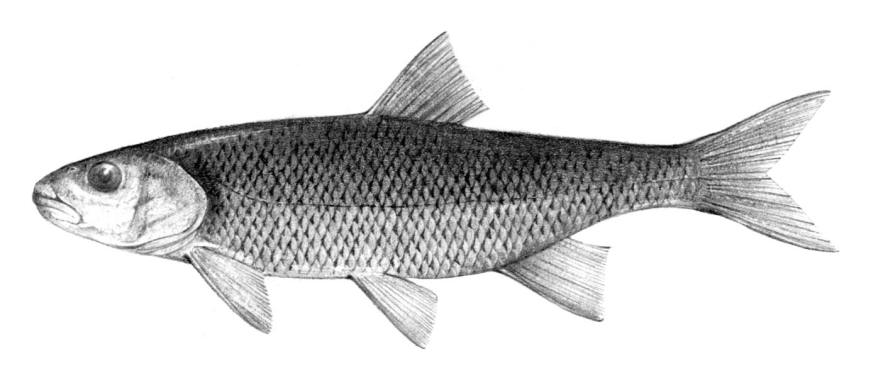
Taxonomy
Other common names include American Chub, Silver Chub, Mohawk, White Chub, Roughnosed Chub, Windfish, Corporal, Chivin, Whiting, Roach Dace and Shining Dace.
Key Characters
This species is distinguished by having protractile premaxillae (a groove between the upper lip and the snout), a flat barbel in advance of the mouth corner in the groove above the upper lip, lateral line scales 43-50, no black spot at the dorsal fin base and each scale base is dark. The barbel may be absent on 1 or both sides in smaller fish.
Description
Dorsal fin branched rays 7, anal branched rays 6-8, usually 7, pectoral rays 15-19 and pelvic fin rays 7-9. Short gill rakers number 6-8. Pharyngeal teeth 2,5-4,2, 2,4-5,2 or 2,4-4,2. Males have small nuptial tubercles on the snout, over the eye, in a patch on the postero-ventral edge of the opercle and on the branchiostegal rays, fine tubercles on the opercular flap and on up to 9 pectoral fin rays.
Colour
The back is olive-brown to blackish, flanks are silvery, occasionally with purple to blue tinges, and the belly is white. There is a dark bar along the shoulder girdle at the edge of the gill opening. Scales above the lateral line are outlined with pigment. Young fish have a wide flank stripe running from behind the eye to the tail, ending in a spot at the tail base. Breeding males have a darkened back and pink opercles and pectoral fins. Peritoneum silvery with darker speckles.
Size
Reaches 51 cm and 1.7 kg. Bernatchez and Giroux (2000) report the world angling record as a 1.7 kg fish caught in in Maine in 1986. A 1.06 kg fish was caught in the York River, Ontario.
Found from New Brunswick to southern James Bay drainages of Québec and Ontario south to Lake Erie through eastern Ontario and east of the Appalachian Mountains to Virginia.
Origin
This species entered the NCR from an Atlantic coastal refugium.
Habitat
Fallfish live in clear streams and rivers with gravel or rock bottoms, and less frequently in lakes. They are found at the base of falls or rapids and in slower water.
Age and Growth
Life span is estimated to be 10 years. Males grow faster than females after age 4. Males mostly mature at age 3 and females at age 4.
Food
Food includes aquatic insects, terrestrial insects taken at the water surface, crustaceans and fishes. Kingfishers and mergansers are known to eat them.
Reproduction
Spawning takes place in May in eastern Québec at 16.6°C. Post-spawning males with tubercles scars have been caught in the NCR on 13 July. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 3 April to 20 May at 9-14ºC. Males begin nest building generally at 12°C, carrying stones by mouth. Nests can be 92 cm high and 183 cm across and are elongate in flowing water or domed in quiet water. A nest can weigh up to 81.8 kg. Nests can be in midstream or at stream edges protected by overhanging vegetation. Common shiners may use the nest and hybrids result. Eggs are adhesive after shedding and 2.7 mm in diameter, and are covered with gravel by the male after deposition. A female may have up to 12,321 eggs. This species is unusual among its relatives because communal spawning occurs. Several fish of both sexes are found over nests. Males establish a hierarchy among themselves by parallel swimming, lateral displays, head butting and tail biting. Other fish species are tolerated. A dominant male starts the communal spawning by swimming to the nest with a stone in his mouth and dropping it, or by "rushing" onto the nest from downstream. Other Fallfish are stimulated to rush onto the nest and spawn. There is no clasping of the female as eggs and sperm are shed into the gravel. Other male Fallfish often break up the dominant male spawning couple. Communal spawning enables many males to fertilise eggs without having to construct nests.
Importance
Fallfish can be caught by fly fishing and give good sport but are a nuisance to anglers trying for trout. They may be caught on worms, grasshoppers or dough as well as on flies meant for trout and are good eating.
Catostomidae - Suckers - Catostomes
Suckers, redhorses, buffalofishes and carpsuckers are found in freshwaters of North America with 1 species in the Yangtse Kiang of China and 1 North American species also in northeastern Siberia. There are 68 species with 18 species and 1 subspecies in Canada, and 7 species in the NCR. The record of Erimyzon sucetta (Lacepède 1803) in the NCR by Small (1883) is an error. Suckers are mostly small to medium-sized fishes with a maximum length of 1.0 m.
The family is characterised by the fleshy lips which are variously folded (plicae) or bumpy (papillae), the broad lower lip often has a cleft, the mouth is ventral, sucking and highly protrusible, no jaw teeth, pharyngeal or throat teeth in a single row of 16 or more teeth, up to as many as 190, which grind food against a horny pad at the base of the basioccipital bone as in the Carp Family, barbels and an adipose fin are absent, 10 or more dorsal fin rays, no fin spines, the anal fin is placed far back on the body, scales are cycloid, the upper jaw is bordered by the premaxilla and maxilla bones, there are y-shaped intermuscular bones, the swimbladder has 2-3 chambers and is connected to the gut, and pelvic fins are on the belly (abdominal). Suckers are tetraploids, having two sets of chromosomes numbering around 100.
Suckers are related to the Carp Family. The redhorses (genus Moxostoma) are difficult to identify and require some practice. The redhorses have a swimbladder with 3 chambers while other suckers, such as those in the genus Catostomus, have 2 chambers. Fin ray counts used in identification are the principal, elongate rays. Rudimentary rays, usually 2-4, at the beginning of each fin are not included.
Most suckers are bottom-feeders, grubbing through detritus for food by touch and taste. There are two basic body forms, a heavy, deep-bodied one and a more rounded, stream-lined form. The former are found in large slow rivers and lakes and the latter in faster streams. Young are often plankton feeders with mouths at the tip of the snout. The mouth migrates ventrally with growth and diet switches to bottom foods. Mollusc feeders have heavy, molar-shaped teeth while those feeding on aquatic insects and crustaceans have thin, sharp-edged teeth. Suckers have brightened colours during the spawning season and develop nuptial tubercles. Hybrids are common among suckers. They are not generally eaten in Canada but can be quite tasty, and are of only minor interest to anglers, perhaps unjustly so. However they are very important forage species, fed on by a variety of sport fishes.
The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating redhorse suckers in the Mississippi River, South Nation River and Ottawa River. As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Quillback / Couette
Carpiodes cyprinus (Lesueur, 1817)
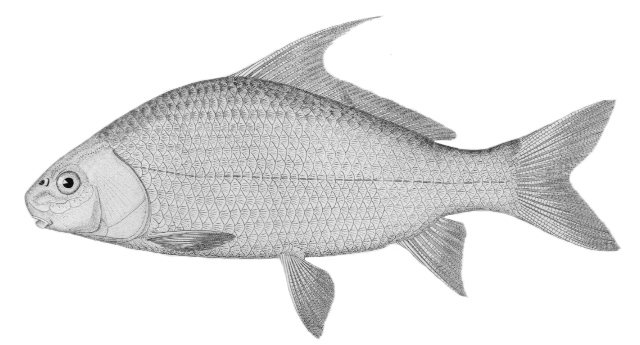

Taxonomy
Other common names include Carpsucker, Lake Quillback, Eastern Quillback, Long-finned Sucker, Broad Mullet, White Carp, Silvery Carp, Drum, Plains Carpsucker, Brême and Poisson à couette.
Key Characters
The Quillback is recognised by the long dorsal fin with 22-32 major rays which has the anterior rays elongated into a high, sickle-shape.
Description
The longest dorsal fin ray is 4-6 times the length of the shortest. The small mouth is overhung by the snout. The caudal fin is deeply forked. The lips have transverse plicae and the lower lip halves meet at an acute angle and have no knob at the tip. Pharyngeal teeth are thin, small and comb-like with pointed tips. The gut is elongate with 6-9 loops. Gill rakers slender, 25-44. Major anal fin rays 6-9, pectoral rays 15-16 and pelvic rays 8-10. Lateral line scales 33-42. Males have nuptial tubercles on the side of the head below the top of the eye level, on top of the head, on the underside of the head to the anterior branchiostegals and on the first dorsal fin ray, first 11 pectoral rays and 8 pelvic rays. Anal and caudal fins are tuberculate in some males. There are up to 18 tubercles per scale on the 3-4 rows above and below the lateral line. There may even be tubercles on the cornea, completely surrounding the pupil. Females may have fewer tubercles than males.
Colour
The back and upper flank are olive to dark or light brown or tan, the flanks are silvery often with a golden yellow tinge, and the belly cream to white. Scales have silver, green or blue reflections. The eye is silvery. The upper fins are dusky, lower fins more transparent, sometimes with basal orange or yellow tinges and a white leading edge. The anterior edge and dorsal margin of the dorsal fin are black. The snout tip and lips are often a milky-white.
Size
Reaches 66.0 cm and 6.18 kg. The world, all-tackle angling record from Lake Michigan, Indiana weighed 2.94 kg and was caught in 1993.
Found in the St. Lawrence River around Montréal, Ottawa River up to Ottawa, Richelieu River, Lake Champlain, lakes Erie and Huron and Georgian Bay, Lake of the Woods, central and southern Manitoba, Saskatchewan and Alberta. In the U.S.A. south to Louisiana and west to the Florida Panhandle, west of the Appalachian Mountains and south to Virginia east of these mountains from Pennsylvania.
Origin
This species entered the NCR from an Atlantic coastal refugium or possibly a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
The Quillback is found in clear and turbid waters of rivers and lakes tolerating warm temperatures as high as 29-31°C although generally its preferred temperature is 22.1°C. Slower water is favoured with sand and silt substrates.
Age and Growth
Life span is 46 years for females and 52 years for males. Maturity is attained at 6-8 years for females at a minimum fork length of 34.5 cm and 4-6 years for males at a minimum fork length of 28.0 cm.
Food
Food is bottom fauna such as aquatic insects, crustaceans and molluscs, macrophytes and any organic matter in bottom sediments.
Reproduction
An upstream spawning migration in the Ochre River, Manitoba takes place once water temperatures reach 5°C but only when discharges are high. Spawning occurs in streams, flooded areas or bays of lakes in April to July, perhaps later to September. Water temperatures are 7-18°C in Manitoba, 19-28°C in other studies. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 29 April to 3 June at 7-18ºC. Eggs are broadcast over gravel in riffles or over sand and even mud, and number up to 360,000 per female.
Importance
This sucker is of minor commercial importance in U.S.A. where it is caught with gill nets, and has white, tasty flesh. Anglers catch them in the U.S.A. by using dough balls, bread, worms and grubs as bait or by snagging.
Longnose Sucker / Meunier rouge
Catostomus catostomus (Forster, 1773)
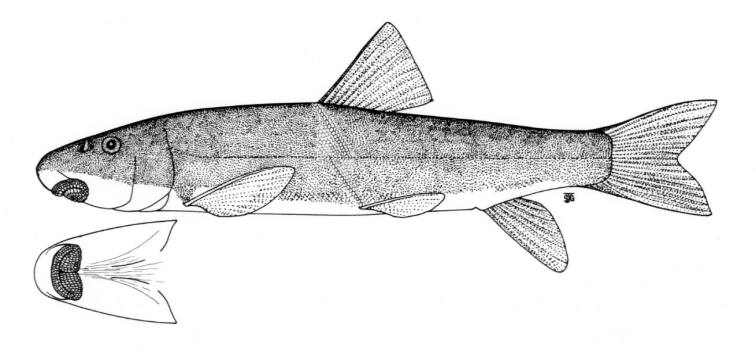
Taxonomy
Other common names include Sturgeon, Sturgeon-nosed Sucker, Northern, Finescale, Red, Black or Red-sided Sucker, Carpe-soldat, Carpe rouge, Milugiak, Nannilik, Miluiak and Miluqiaq.
Key Characters
This species is identified by having 9-11 principal dorsal fin rays, 91-120 scales in a complete lateral line, no membranous stays between the pelvic fins and the body, and the lower lip is completely cleft in the mid-line.
Description
Scales between dorsal fin origin and lateral line usually16-18. Anal fin principal rays 7, pectoral rays 16-18 and pelvic rays 9-11. Gill rakers short, numbering 23-30. Breeding males have nuptial tubercles on the head, anal fin and lower caudal fin lobe.
Colour
The back is dark olive, brown, grey or black fading to a cream or white belly. The scales are outlined with dark pigment on the back and upper flank. Both sexes have a head and mid-flank pink to red stripe bordered below by black and abruptly set off from the light belly when breeding, and males have a black back, females a gold to copper-brown back. The underside of the head is yellow to orange in both sexes and the belly pinkish. Fins are similar to the adjacent body. Peritoneum dusky black. Young may have 3 black blotches on the flank, sometimes forming saddles over the back, but this not as obvious or common as in the White Sucker.
Size
Reaches 64.2 cm and 3.31 kg for a specimen from Great Slave Lake. The world, all-tackle angling record weighed 2.86 kg and was caught in the St. Joseph River, Michigan in 1986.
Found from Labrador and New Brunswick to British Columbia, Alaska and into eastern Siberia. Absent from Nova Scotia and the Atlantic, Pacific and Arctic islands of Canada. In the U.S.A. in states close to the Canadian border and south to Idaho and to West Virginia. This species is not confirmed by specimens from the NCR deposited in a museum, although the species is widely distributed in Québec and Ontario. The record is based on a report by a local resident (J. C. McCuaig, Secretary-treasurer of the Fish and Game Protective Association for the counties of Gatineau, Hull, Papineau and Pontiac) and recorded in Dymond (1939) as from Lac Deschênes in the Ottawa River. Provost et al. (1996) also record it from the Ottawa River at various sites within the NCR. A fossil of Quaternary age (ca. 10,000 years ago) identified as this species has been found in a clay nodule from Hiawatha Park in the NCR (Harington, 1983; McAllister et al., 1987).
Origin
This species entered the NCR from possibly an Atlantic, Beringian or Missourian refugium (Mandrak and Crossman, 1992).
Habitat
This sucker is common in clear northern waters of rivers and lakes but is restricted to cool areas in the south such as deep lakes, as deep as 183 m in Lake Superior. Its preferred temperature is 8-17°C.
Age and Growth
Life span is about 24 years. Females live longer and grow larger than males. Maturity is attained at 2-10 years. There is a wide range in growth and in maturity patterns, reflecting the broad distribution of this species and the various habitats. Growth tends to be slower and life span longer in the north. However growth in Great Slave Lake is faster than in smaller, southern lakes. Some populations are dwarfed.
Food
Food is crustaceans, aquatic insects, clams, worms, and plant material. Algae may comprise up to 95.5% of gut contents in medium-sized suckers of the Matamek River, Québec and consume about 2% of the annual periphyton production in the river. Once regarded as an egg predator of sport fish, this is now discounted. It is commonly eaten by other fishes, birds, bears and other mammals.
Reproduction
Spawning takes place in streams or lake shallows once water temperatures exceed 5°C. This is usually April-July in Canada and the spawning run peaks several days before that of White Suckers. The spawning process may only extend over 5 days. Males remain on the spawning grounds longer than females. Most fish migrate in the evening but spawning on gravel bottoms occurs during the day. The males occupy faster water near the stream middle, females slower water at the edge. A female approaches the males and is flanked by 2-4 of them. Eggs and sperm are shed as males clasp the female with their pelvic fins or vibrate against her with the anal fin. Each thrashing and splashing spawning act lasts only 3-5 seconds but is repeated up to 40 times an hour. The eggs are white and adhere to the gravel. Each female may have up to 60,000, 3.0 mm diameter eggs in her ovaries. Fry emerge from the gravel after 1-2 weeks and when 1.0-1.2 cm total length start to migrate downstream during nights.
Importance
This species has been used for food for humans and dogs. The flesh is good eating, being white and flaky. It is excellent smoked. Great Lakes catches are sold as "mullet" with other suckers.
White Sucker / Meunier noir
Catostomus commersonii (Lacepède, 1803)
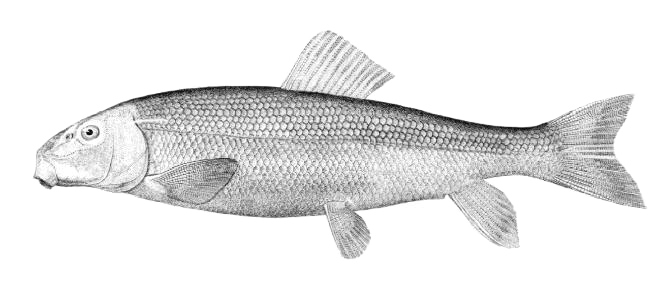


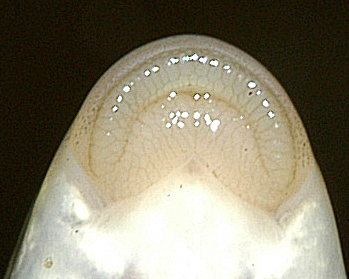








Taxonomy
Other common names include Common, Common Brook, Coarse Scaled, Fine Scaled, June, Eastern, Mud, Slender, Grey or Black Sucker, Black Whitehorse, Mullet, Carp, Carpe noir and Catostome commun noir.
Key Characters
This species is recognised by having 53-85 (usually 58-68) scales in a complete lateral line, 9-14 principal dorsal fin rays, a completely cleft lower lip which is wider than deep, and no membrane connecting the pelvic fins to the body.
Description
There are 8-11 scales between the dorsal fin origin and lateral line. Principal anal rays 6-8, pectoral rays 15-18 and pelvic rays 10-11. Gill rakers short and numbering 20-27. The numerous pharyngeal teeth are comb-like except the lower 5 which have wide crowns. Males have large nuptial tubercles on the anal fin, lower caudal fin and the caudal peduncle. The tubercles follow the fin rays and may branch with them with up to 4 rows on lower caudal rays. Smaller tubercles are found on the head and belly, number 1-3 on the rear margin of back and upper flank scales and line the rays of the dorsal and pelvic fins.
Colour
The back and upper flanks are grey, dusky olive, brassy, brown or almost black, flanks are greenish-yellow to brassy and the lower flank and belly are creamy to white. Scales on the upper flank and back are outlined with pigment. The anal fin is white and other fins are dusky. Pectoral, pelvic and anal fins may have an orange tinge and the pectoral and pelvic fins may have a white leading edge. Spawning fish are more golden on the flank and darker on the back. Males have a pink to scarlet or blackish lateral stripe. Young fish have 3-4 obvious blotches along the flank. Peritoneum pale. Faber (1984c) illustrates a larva.
Size
Reaches 76.0 cm total length, and reputedly 17.5 kg. The world, all-tackle angling record weighed 2.94 kg and was caught in the Rainy River, Minnesota in 1984. A fish from Lake Joseph, Ontario weighed 2.5 kg.
Found across Canada; absent only from Newfoundland, P.E.I., Gaspé, the margins of Labrador, the N.W.T. (except for the prairie border area and the Great Slave and Mackenzie River basins), absent from most of the Yukon and from southern and western British Columbia. In the U.S.A. south to Georgia and New Mexico.
Origin
This species entered the NCR from possibly a Mississippian, Missourian or an Atlantic coastal refugium.
Habitat
White Suckers are found in large rivers, and in shallows of lakes, but may descend to about 50 m, and in rivers and streams tributary to lakes. There is usually some vegetation, and the bottom is mud although boulders or sand forms part of the habitat. These suckers are very tolerant of polluted and muddy waters. Their preferred temperature is 22.4°C.
Age and Growth
Maximum life span is about 25 years. Fish in the Gatineau River, just north of the NCR, live over 10 years and generally grow faster in the lower reaches of this river (Gagnon et al., 1995; Couillard et al., 1995; Couillard and Hodson, 1996). Maturity in the Gatineau varied between 4-6 years, later above Maniwaki than below, suggesting a richer environment lower down this river. Pectoral fin ray sections are used in aging White Suckers as scales give underestimates of age. Annual mortality in the Laurentides, Québec is 90% for 7 year old and older fish. Females grow faster, live longer and are larger than males. Sexual maturity is reached at 2-9 years, varying with locality across Canada and sex (males mature 1 year younger than females; in Saskatchewan males matured at 6-7, and females at 6-9; in Alberta at 2 and 3 years respectively, although first spawning was at 5 and 6 years). A dwarf or stunted population near Chicoutimi, Québec matured at 2-3 years and had an 8 year life span. Dwarf populations are known to co-exist with normal sized suckers but in one case at least these are large females and small males, the size difference compounded by a higher rate of male mortality.
Food
Food is aquatic insects, crustaceans, molluscs, some phytoplankton and sometimes eggs of other fishes although they are not now considered to be a major predator on sport fishes. Water fleas are often a major diet component. Young suckers feed on plankton until about 1.8 cm long when the mouth moves to a ventral position and bottom feeding is adopted. Most feeding occurs at dawn and dusk. These suckers are very numerous and an important food for a wide variety of sport and other fishes, birds and even bears.
Reproduction
Spawning occurs usually in May to July in Canada after a migration into gravelly streams or lake margins at 10°C or warmer. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 29 April to 27 May at 7-13.5ºC. In a stream tributary to Lac Philippe, Gatineau Park water temperature was 8.5°C on 14 April 1986 and no fish were seen but by 18 April the temperature was 13°C in an unusually warm spring and a run occurred (Coad, 1988). In previous years fish were observed concentrating in pools at precisely 10°C in early May. The fish swim up a concrete drainage slope into a culvert, with their bodies mostly out of the water which is flowing at 2 m/sec. Lake spawners may clean the gravel before spawning. Spawning in a quarry next to the Canadian Museum of Nature building on Pink Road, Gatineau occurs in the first half of May (personal observations, 1998 and succeeding years). Ospreys may take these fish when spawning as one osprey was observed in the area with a large fish in its talons and the spawning fish were nowhere to be seen (5 May 2004, personal observations, Michel Gosselin, pers. comm.). Males arrive on the spawning ground 2-3 days before females. Most eggs are shed at night with much splashing in the shallow water of riffles. The quarry fish can be observed spawning in daylight, with their bodies partly out of the water at the quarry's edge. Each female is pressed against by 1-10 males, using their tuberculate anal and caudal fins. The dorsal fin is held erect, pectoral fins are spread, the tail lashes the water and the head is shaken. Usually the female is flanked by a male on each side. Spawning only takes 1.5-4 seconds but occurs 6-40 times an hour and the season lasts up to about 2 weeks. Each female can carry up to 139,000 yellow eggs with a diameter up to 3.0 mm. Eggs hatch in 1-2 weeks depending on temperature, larvae are 8.0-9.0 mm long and the fry migrate to the lake after spending 1-2 weeks hiding in the gravel. There is an estimated spawning mortality of 16-47% depending on the population and its predators. Females do not spawn every year but the frequency of spawning increases with age. Males are 3 times as likely to spawn in a given year than females. Nuptial tubercles are lost shortly after spawning.
Importance
This sucker has white, flaky flesh and is quite tasty. The spring spawning run offers an opportunity for dip-netting and considerable numbers are eaten locally across Canada. It has been marketed with other suckers as "mullet" but has never formed a major fishery. White Suckers are not fished for but can provide good sport taken on light tackle using various baits, wet flies or spinners. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi River, Rideau River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Young White Suckers are sold as bait in Canada for Smallmouth and Largemouth Bass, Northern Pike and Muskellunge. In the U.S.A. they are reared artificially and in both Canada and the U.S.A. are used for feeding hatchery sport fishes. In the past, Canadian farmers pitch-forked wagon-loads of suckers out of streams to use as pig food. Introduced populations significantly decrease the production of Brook Trout.
Silver Redhorse / Chevalier blanc
Moxostoma anisurum (Rafinesque, 1820)
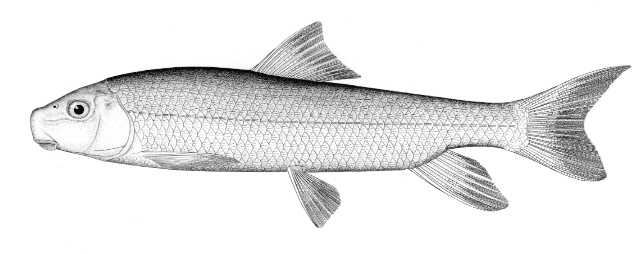

Taxonomy
Other common names include Suceur blanc, Silver Mullet, Whitenose Redhorse, White Nosed Sucker, Chevalier blanc, Carpe blanche and Moxostome blanc.
Key Characters
This species is distinguished by a short dorsal fin, a swimbladder with 3 chambers, lateral line scales 38-48, scales around the caudal peduncle usually 12, occasionally 13 (rarely as high as 15-16), greatest body depth enters body length to end of scales 3.5 times of less, the lower lip is thin and the plicae are long and narrow with cross lines.
Description
The mouth is overhung by the snout. The dorsal fin edge is convex in adults to straight in young. The upper lip is narrow and has plicae only on the inside. The lower lip is deeply cleft and the halves meet at 90° or more. Gill rakers short numbering 25-28. Pharyngeal teeth are elongate and thick but not molar-shaped. The gut has 5 coils. Dorsal fin rays 13-18, anal rays 7, pectoral rays 16-20 and pelvic rays 8-10. Males have large nuptial tubercles on the anal and caudal fins, and minute tubercles on paired fins, dorsal fin, head and on some scales.
Colour
The back is olive-green to bronze or brown with silvery or yellowish-gold flanks and a silvery to white belly. The flank may have bronze tinges particularly in spawning fish. The snout is often white. Scales are outlined with dark pigment but bases are not strongly pigmented. Paired fins are dusky to pale red, orange or yellowish. Caudal and dorsal fins are slatey to dusky. Young have 2-3 flank blotches. Peritoneum silvery with a few black spots.
Size
Reaches 74.0 cm and 6.75 kg, possibly 10.8 kg. The world, all-tackle angling record from Plum Creek, Wisconsin was caught in 1985 and weighed 5.18 kg.
Found in central North America from southern Canada south to northern Alabama and Georgia. In Canada it is found from the upper St. Lawrence River basin in southwestern Québec, west through the Great Lakes and southern Ontario to southern Manitoba, Saskatchewan and over the Alberta border in the North and South Saskatchewan rivers.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
This redhorse is usually found in slow, deep pools of streams and large rivers but also occurs in lakes. It is sensitive to siltation and pollution. Campbell (2001) in a study directed to Moxostoma species found this species to be the second most abundant species after Rock Bass in the Mississippi River and in the lower Gatineau River on 2 June 1998 fishing from 2000 to 2400 hours for Moxostoma only, the second most abundant after M. carinatum with M. macrolepidotum next.
Age and Growth
Life span is 27 years (Reid, 2008) with maturity attained at 5-8 years. Females grow faster and live longer than males. The average fork length in the Lac des Chats (an Ottawa River section) was 489.9 mm and average weight was 519 g (Haxton, 1999).
Food
Food is aquatic insects, crustaceans and occasionally molluscs and phytoplankton.
Reproduction
An upstream spawning run may begin in Lake Erie as soon as the ice goes out. Spawning occurs in May-June at 13°C or more in moderate to fast, turbid, shallow streams with gravel bottoms at less than 2 m depth. Males arrive on the spawning grounds before females and are thought to defend a territory. Eggs number up to 36,340. Comtois et al. (2006) give 3-31 May as the capture period of fish in stage V (spawning) in the lower Gatineau River at temperatures of 9-17ºC.
Importance
It may form part of the commercial catch in the St. Lawrence River.
River Redhorse / Chevalier de rivière
Moxostoma carinatum (Cope, 1870)
Taxonomy
Other common names include Suceur ballot, River Mullet, Greater Redhorse, Redfin Redhorse, Pavement-toothed Redhorse, Big-jawed Sucker, Big-toothed Redhorse, Ballot and Chevalier ballot.
Key Characters
This species is distinguished by a short dorsal fin, a swimbladder with 3 chambers, lateral line scales 41-47, scales around the caudal peduncle usually 12-13, (rarely 15-16), lower lip at least 3 times wider than upper lip and almost straight along rear edge and without transverse lines or papillae across long, narrow plicae, pelvic fin origin anterior to midpoint of dorsal fin base, pharyngeal teeth molar-shaped and numbering 6-9 on lower half of tooth row. This species is difficult to identify in the field and this lack of accurate identification may impair its survival through a lack of awareness of its presence and hence biology (Parker, 1988).
Description
The mouth is slightly overhung by the snout. Gill rakers 26-30. Dorsal fin rays 12-15, anal rays 7-9, pectoral rays 15-19 and pelvic rays 8-10. Gut with 5-6 coils. Males have nuptial tubercles on the back of the head, snout, cheek and throat. Minute tubercles line scale margins on the upper flank, on dorsal, anal and caudal fin rays and on the anterior pectoral and pelvic fin rays. The anal fin is longer in males than females. Females have thickened skin on the lower caudal peduncle.
Colour
The back and upper flank are brown or bronze to olive- or lime-green, paling to a white to yellow belly. The flank may be silvery. There is often a golden sheen over the flank and belly. Dorsal, anal and caudal fins are pale red, leading edges of the pelvic fins are pale red, and of the pectoral and pelvic fins are white. Pectoral fins olive. There may be a thin vertical line at the caudal fin base. Upper flank scales have a dark pigment spot at their base. Breeding males have a head and mid-flank dark stripe ending above the anal fin. Peritoneum silvery.
Size
Reaches 79.8 cm total length, and 7.938 kg (Gatineau River, Campbell (2001), the longest and heaviest on record, although general records cite 90.0 cm). The world, all-tackle angling record from Limestone Creek, Alabama weighed 2.52 kg and was caught in 1985. A 3.96 kg fish is recorded from the Trent River, Ontario.
Found in the St. Lawrence River and nearby waters around Montréal, the Ausable River of Lake Huron and the Grand River of Lake Erie in Canada. South to Florida and west to Ohio and Arkansas but sporadically distributed. In the NCR it is found in the Mississippi River and the nearby Ottawa River. Parker (1988) suggests that other populations exist in the Ottawa River, confirmed by Moisan (1998) and Campbell (2001). Moisan (1998) gives several additional map points in the Ottawa-Gatineau downtown area of the Ottawa River. The Mississippi and Gatineau River populations are some of the few known reproducing in Canada.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
River Redhorses, as the name suggests, are found in moderate to large, fast rivers in deeper water when not spawning. They can occur in lakes, though less frequently. They are found over a silt-free stone, rubble or bedrock bottom with a preference for watersheds dominated by limestone or shale bedrock. Low turbidity is favoured (McAllister et al. (1985). Spawning requires rock, stone, gravel and cobble substrates in fast-flowing and shallow water (often less than 1 m deep). Mean July water temperatures are greater than 20°C with a maximum at 25°C (Parker, 1988; McAllister et al., 1985). Campbell (2001) caught this species in the Mississippi and Gatineau rivers from 7° to 27°C. They are soon eliminated by pollution and a population in the Mississippi River of the NCR may be declining, probably due to sport fishing (Parker, 1988), but also due to increased sediment load from agriculture affecting the invertebrate food supply (Campbell, 2001). Parker and McKee (1980) suggest that it makes up 5% of redhorses captured in the Mississippi River. However see the data from Campbell (2001) summarised in part above under M. anisurum where the River Redhorse accounted for 4.7% of the total sample of fishes in the Mississippi River and 12.1% of the Catostomidae although sampling methodology is probably the reason for the disparity. This study also showed that in the Gatineau River from 29 April to 23 June 1999, M. carinatum comprised 11.4% of all fish captured and 32% of all Catostomidae. The population in the Mississippi River between Almonte and Galetta numbers an estimated 623-830 fish, less in other sections, while the Gatineau River spawning population near the Alonzo Wright Bridge ranged from 671-694 fish.
Age and Growth
Life span is at least 30 years (Campbell, 2001) with maturity at 5-10 years. Both males and females exceed 70 cm in the NCR (Campbell, 2001). Growth rate for Ontario fish is slower than in Missouri populations. In the Mississippi River study of Campbell (2001) males were shorter and less heavy than females but this was not the case for fish from the Ottawa River. The average fork length in the Lac des Chats (an Ottawa River section) was 620 mm and average weight was 3531 g (Haxton, 1999). Young are smaller than other redhorses because of the later spawning.
Food
Food is aquatic insects such as mayflies, trichopterans, chironomids and coleopterans and, when larger, molluscs crushed by the molar teeth, as well as other bottom invertebrates such as crayfish. Food is detected by sight.
Reproduction
Spawning may occur in large rivers or in the upper parts of large tributary streams after a migration. Canadian reproduction probably occurs at the end of May and into June at water temperatures over 18°C. River Redhorse spawn after the Silver and Shorthead redhorses in the lower Gatineau River (Comtois et al., 2006). Campbell (2001) summarised spawning in the lower Gatineau River in 1999 as follows. Fish arrived on the spawning grounds between 30 April and 10 May at temperatures of 7.5-12.5°C, males leaking milt were observed between 20 May and 30 May at temperatures of 12.5-14.5°C, females leaking eggs were observed between 30 May and 9 June at temperatures of 14.5-18.0°C, spawned females were observed between 9 June and 19 June at temperatures of 18.0-19.0°C and spawned males were seen around 19 June. The spawning period is mostly in late May and June at 17.5-19.0°C. The water is fast and shallow, about 1.5-2.5 m deep (Campbell, 2001). Males arrive on gravel shoals before females and excavate nests using tail sweeps, head pushes and carrying with the mouth. This may occur only where the spawning substrate is small and, some of it at least, is a result of spawning activity, not a preparation for spawning (Campbell, 2001). Comtois et al. (2006) give similar spawning dates for the lower Gatineau River (10 May-23 June at 12,5-19.0ºC). In the NCR, the large rocks and stones (>7 cm) in the Mississippi and Gatineau rivers may well make redd construction difficult. Nest or spawning areas may be up to 2.4 m across and 30 cm deep. A male maintains a stationary position over the nest facing upstream until a female approaches when he darts back and forth across the nest. A second male joins the first synchronising his movements with the first until the female takes up a position between the 2 males. All 3 fish vibrate strongly, yellow eggs are shed and fertilised, and buried in the gravel. Eggs are up to 4.4 mm in diameter, non-adhesive, and each female can have up to 42,630 eggs. Incubation is short, 3-4 days at 22°C (Parker, 1988).
Importance
This species has formed a minor part of a commercial catch near Montréal in the past. It may be caught by sport fishermen in Ontario. Bone remains have been found in Indian middens. The Committee on the Status of Endangered Wildlife in Canada gave this species a status of "Special Concern" in 1987 (COSEWIC, 2002). The proposed micro-hydro power facility on the Mississippi River at Blakeney Falls was a potential threat to this species, averted by local protests (Chaundy, 1987). Nearly 70% of fish examined by Campbell (2001) in the Mississippi River had lacerations, infections, leeches, fungus, tumours, blindness and invertebrate parasites while only 6% exhibited similar trauma in the Gatineau River. Lamprey scars were noted on the Gatineau River fish (no lampreys are known from the Mississippi River).
Shorthead Redhorse /
Chevalier rouge
Moxostoma macrolepidotum (Lesueur, 1817)

Taxonomy
Other common names include Suceur rouge, Northern Redhorse, Common Redhorse, Redfin, Red Sucker, Common Mullet, Short-headed Mullet, Bigscale Sucker, Brook Mullet, River Sucker, Eastern Redhorse, Chevalier rouge, Carpe à cochon aux ailes rouges and Moxostome doré à cochon.
Key Characters
This species is distinguished by a short dorsal fin, a swimbladder with 3 chambers, lateral line scales 38-48, scales around the caudal peduncle 12-13 (rarely 15-16), lower lip thick, posterior edge nearly straight and lips not nearly as wide as snout, pharyngeal teeth not molar-like but blade-like, pelvic fin origin opposite dorsal fin mid-point, eye large (diameter about two-thirds or more of lip width), nostrils behind maxillary tip, and a small mouth overhung by snout.
Description
The dorsal fin is emarginate and appears s-shaped at its posterior margin. The front of the head is rounded and subconical. Gill rakers 22-30. Dorsal fin rays 10-15, anal rays 6-8, pectoral rays 14-19 and pelvic rays 8-10. The gut has 6 rounded coils. Males have large nuptial tubercles on the anal and caudal fins with minute tubercles scattered over much of the rest of the body and fins.
Colour
The overall colour is brown to olive fading to a whitish or yellowish belly. Scales on the upper flank have black pigment at their base forming a crescent. Variously tinged with reflective golden, bronze, yellow or green. Fins are all reddish to some degree. Pectoral and pelvic fins may be more orange or yellowish, the dorsal fin may have only a red tip with a black margin and dusky to orange over most of the fin and the caudal fin may only be margined with red. Young fish have 2-4 dark saddles. Breeding males and females have blood-red caudal fins and lower fins are a brilliant orange-red. Peritoneum silvery.
Size
Attains 75.0 cm and 5.75 kg. The world, all-tackle angling record weighed 2.16 kg and was caught at Island Park, Indiana in 1984. A 4.0 kg fish is recorded from the North River, Ontario.
Found from southwestern Québec and its James Bay shore, throughout Ontario, southern and central Manitoba and Saskatchewan, and southeastern Alberta. In the U.S.A. south to South Carolina, Alabama and Texas and west to Montana. This is the commonest redhorse in the NCR.
Origin
This species entered the NCR possibly from an Atlantic or Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
This redhorse is usually found in lakes and the clearer rivers with slow to moderate current, over sand, gravel or rock. It is not very tolerant of pollution and siltation but can withstand water temperatures up to 37.2°C and so is found in sluggish, shallow and unshaded rivers. Its preferred temperature is 26.0-27.5°C. In the NCR this species is found in some polluted waters and where river bottoms are covered in wood chips (McAllister and Coad, 1975). In the South Nation River from the mouth to the weir at Plantagenet Springs, this species was the one most commonly caught in hoop-nets (Lauzon, 2003).
Age and Growth
Life span in Canada is cited as 14 years, longer than southern populations. Reid (2008), using opercles for ageing, found life span to be 20 years. Maturity is attained at 2-5 years depending on locality. Near Montréal life span is reported to be 11 years with maturity attained at age 3-6. In the Plantagenet and Chesterville reaches of the South Nation River, an age range of 2-10 years was recorded (Lauzon, 2003).
Food
Food is principally aquatic insects and crustaceans, with worms and molluscs and some phytoplankton slurped and strained from bottom sediments.
Reproduction
Spawning takes place from May to July, mostly May in Canada, after a migration into riffle areas of streams at about 11°C around Montréal. A female caught above the Deschênes Rapids of the Ottawa River on 24 May had eggs at maximum size. Nuptial tubercles were well-developed on a fish recorded by F. W. Schueler on 28 April 1990 at Oxford Mills Dam on Kemptville Creek. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 29 April to 21 June at 7-19ºC. Males arrive first and defend a territory on spawning grounds although no nest is built. Depressions seen in bottom sediments may be incidental to spawning movements and not a nest. Each female is flanked by 2 males who press their caudal regions against her. Spawning takes only 2-3 seconds and is accompanied by vibrations. Eggs are shed and abandoned at night, in the early morning, or during the day. Each female may carry up to 44,000 eggs of 2.2 mm diameter.
Importance
This is the most abundant redhorse species in Canadian waters and was important in commercial redhorse catches, although these catches were minor compared to other fishes. The flesh is good eating, reputedly the best of all suckers but, as with all redhorses, there are numerous bones which make them difficult to prepare. The flesh should be scored or turned into ground meat for patties. Environnement Québec has a recommended limit of 8 meals per month for small fish and 4 for medium and large fish from the Lièvre River at and above Buckingham (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version. Anglers catch this species on spawning migrations on hooks baited with worms, grasshoppers, grubs and meat, and on artificial wet flies and plugs.
Greater Redhorse / Chevalier jaune
Moxostoma valenciennesi Jordan, 1885
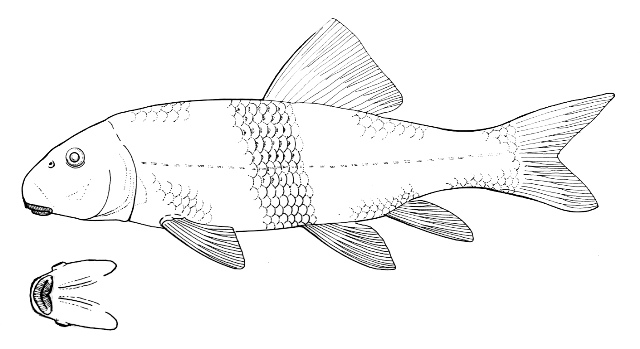

Taxonomy
Other common names are Suceur jaune, Common Redhorse, Carpe jaune and Moxostome jaune.
Key Characters
This species is distinguished by a short dorsal fin, a swimbladder with 3 chambers, lateral line scales 41-46, scales around the caudal peduncle usually 15-16, scales over the back just in front of the dorsal from lateral line to lateral line usually 12-13 (excluding lateral line scales), and pharyngeal teeth small, comb-like and 12-30 on lower half of the tooth row.
Description
The maximum body depth enters body length to the end of the scales 4 times or more. The dorsal fin usually has a convex (straight or rounded) margin. The snout is longer than the postorbital length. The mouth is not overhung by the snout. The upper lip is wide with coarse plicae. The lower lip is deeply cleft and wide, plicae are coarse, deep and lack cross striations. Gill rakers 24-31. Dorsal fin rays 11-15, anal rays 7, pectoral rays 15-19 and pelvic rays 8-10. The gut has 5-8 coils. Males have nuptial tubercles on the anal and caudal fins and also on the pectoral and pelvic fin rays. Anal fin tubercles may be up to 5 mm across at the base and are larger than those on the caudal fin. The head and body scales are also minutely tuberculate.
Colour
Overall colour is dark olive-green, or greyish, with bronze, yellow-gold or golden tints on the flanks, fading ventrally to a milky-white or silvery-white belly. The overall colour may be a golden-brown. The dorsal, anal and caudal fins are dark red. The caudal may be mostly grey. Median fins may be red only at their margins. Scales have a dark crescent of pigment on the base. The anterior tip of the dorsal fin is white to pink and the anterior rays of the pelvic and pectoral fins white, yellowish or reddish-orange. The pectoral fin is olive and the pelvic fin is pale red. This species is darker overall than the Silver Redhorse with which it is commonly caught.
Size
Reaches 80.0 cm total length and 7.3 kg. The world, all-tackle angling record weighed 4.16 kg and was caught in the Salmon River, New York in 1985.
Found in southern Ontario and southwestern Québec in the St. Lawrence River, Richelieu River and Ottawa River, lakes Ontario, Erie, St. Clair and Huron and their tributaries. In the U.S.A. in the southern Great Lakes, St. Lawrence River and upper Mississippi River basins.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
This redhorse favours clear, clean, hard-bottomed streams and lake margins but also occurs over softer bottoms in muddy areas. It may be sensitive to turbidity, siltation and pollution. It may be generally uncommon or disjunctly distributed but this is confounded by identification problems.
Age and Growth
Life span is 20 years with sexual maturity attained at 5-9 years, usually one year later for females than males. In the Chesterville Reach of the South Nation River an age range of 2-12 years was recorded (Lauzon, 2003).
Food
Food is principally crustaceans, molluscs and aquatic insects, with some macrophytes and worms. Eggs of this redhorse are eaten by American Eels, Fallfish and Yellow Perch, and by the redhorses themselves while on the spawning grounds.
Reproduction
Spawning occurs over a 5-14 day period from mid-May to early July at 13.0-19.9°C, often after an upstream migration to suitable spawning habitat. Sunny afternoons are favoured for spawning although it may occur as late as midnight. There are up to 71,920 eggs per female up to 2.0 mm in diameter. A female from the Rideau River in the NCR had eggs up to 2.0 mm on 16 June. The spawning habitat is less than 2 m deep and has a pebble, gravel, cobble, sand or rubble bed and moderate to swift current. Males move slowly on the spawning ground, often gently nudging and pushing each other. The female selects a spawning site and is flanked by 2 males. Other, satellite males attempt to join in. The female is nuzzled by males in the genital area. Eggs and sperm are shed as the fish quiver and tremor for 2-8 seconds, the males pressing against the female. Dorsal and caudal fins will break the water surface during spawning and males roll over one another and the female. One study in southern Ontario showed males maintain station on or near spawning areas and 2-3 males attempt to initiate spawning when an individual female approaches from downriver.
Importance
It may form a small portion of commercial catches of coarse fishes and is occasionally caught by anglers.
Ictaluridae - North American Catfishes - Barbottes et Barbues
Bullhead or North American freshwater catfishes are found from southern Canada to Guatemala. There are about 45 species with 10 recorded from Canada and 6 in the NCR.
This is the only catfish family in Canada and is easily recognised by the scaleless skin, 8 barbels (2 on the nostrils, 2 at the upper jaw corners and 4 on the chin) and the dorsal and pectoral fins with a spine at the front. There are usually 6 dorsal fin soft rays. The palate lacks teeth. An adipose fin is present. In the United States some members of this family exceed 1.3 m and 45 kg.
They are related to the Carp and Sucker families which also have the chain of bones connecting the swimbladder to the ear. Important identification features are the nature of the pectoral spine and the number of anal rays. Ray counts include all rays, even rudimentary ones and a needle and some dissection may be required. Also important are pore numbers on the head and colour pattern.
Bullheads show a variety of interesting behaviours. All species construct nests and protect their young, although some smaller species take advantage of pollution by nesting in beer cans. Some species can recognise other fish individually by smell and even determine their social status. Chemical "strangers" are subject to aggression. Home sites are also recognised by substrate chemical marks applied by the fish. Territories may be marked chemically in this way. The nostrils are very sensitive to pheromones, hormones released into the water in urine, from the skin and from various glands. The barbels and skin are taste- and touch-sensitive and used to detect food. This is particularly useful in muddy water and at night. Many of these catfishes are nocturnal. "Taste" can also be used in breeding behaviour and in schooling. A Weberian apparatus, modified vertebral bones connecting the brain and swimbladder, are used to transmit sound and bullhead hearing is excellent. Some species can be trained to respond to names!
Catfish lack scales and are aged by making cross sections of pectoral spines to read growth rings. The fin spines can be very toxic because of poisonous cell secretions on the sheath of the spine. Wounds can be painful and extremely swollen for days although in most cases the wasp-like sensation fades after an hour and is gone in about 4-5 hours. Madtoms are named for their hyperactive, darting and dashing behaviour and perhaps stinging effect but even the bullheads (Ameiurus and Ictalurus species) can inflict an uncomfortable, poisoned wound. The spines are an effective defense mechanism for these catfishes but are not fatal to humans. All catfishes should be handled with care. The spines in the dorsal and pectoral fins can be locked in an erect position by an unique arrangement of muscles attached to the spine and associated bones. Bullhead catfishes are hardy and very tolerant of domestic pollution. Larger catfishes, such as the Channel Catfish, are commercially important in the U.S.A. and are cultured in ponds. Catfish restaurant dinners are a specialty in the southern U.S.A. and catfish figure prominently in fish and chips. These fishes are also sought after by anglers, being strong fighters and good eating. Smaller specimens and species have been used in the aquarium trade. They have been introduced outside North America.
A map record for Ameiurus melas (Rafinesque, 1820)(Black Bullhead, Barbotte noire) in the NCR by Mandrak and Crossman (1992) needs confirmation.
Yellow Bullhead / Barbotte jaune
Ameiurus natalis (Lesueur, 1819)
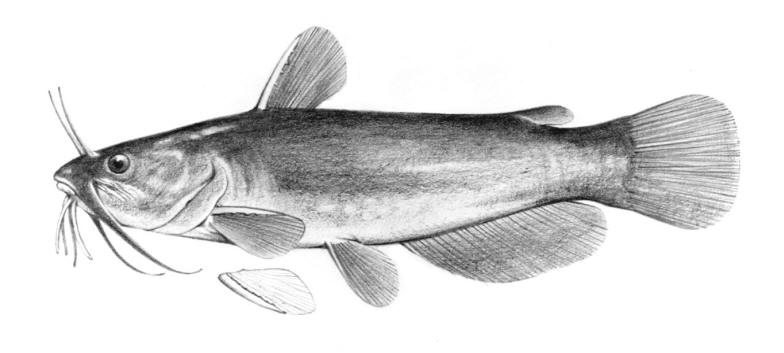

Taxonomy
Other common names include Northern Yellow Bullhead, Yellow Catfish, Creek Cat, White Whiskered Bullhead, Greaser, Polliwog, Yellowbelly Bullhead, Butterball, Buttercat, Paper Skin, Slick Bullhead and Mississippi Bullhead. Formerly in the genus Ictalurus.
Key Characters
This species is identified by the posterior tip of the adipose fin being free of the back, the caudal fin is not deeply forked, mouth corner barbels are about twice as long as nostril barbels, there is no bony ridge between the head and the dorsal fin, the anal fin touches the caudal fin when pressed to the body, chin barbels are yellow to white or pinkish, upper barbels are yellow to grey and the caudal fin is rounded.
Description
Dorsal fin soft rays 6, total anal rays 24-28, pelvic rays 8 and pectoral rays 7-8. The barbs on the dorsal fin spine are very weak. The pectoral spine has strong barbs in young fish but these do not grow with the fish and are weak in large fish. Gill rakers 12-15.
Colour
The back and top of the head are olive-brown to blackish, sometimes grey or even yellow, the flanks are yellowish without mottles and the belly is yellow to white. Fins are brown or olive to dusky with the thicker bases darker. The anal fin may have a central stripe. The adipose fin tip may be pale. Young fish are jet black above and white below with white chin barbels.
Size
Reaches 46.5 cm. The world, all-tackle angling record weighed 1.92 kg and was caught in 1984 in Mormon Lake, Arizona.
Found in southern Ontario and Québec from the St. Lawrence River basin west through lakes Ontario, Erie, St. Clair and southern Huron to North Dakota and south to the Gulf of Mexico. It may be more common in the NCR than records indicate as it is often confused with the Brown Bullhead (Phelps, 2001). Conversely, Brown Bullheads have been mis-identified as this species. Desroches et al. (2008) record this species from Rivière La Pêche and the discharge of Lac Gauvreau in La Pêche Municipality (not on map here).
Origin
This species entered the NCR from possibly an Atlantic coastal or a Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
Yellow Bullheads are found in still or slow waters of lakes, ponds and rivers where there is a lot of vegetation and sand to silt bottoms. They may be found in slow riffles. Their preferred temperature is 28.3°C. They are tolerant of some pollution compared to Black and Brown Bullheads but less tolerant of turbidity. These bullheads are active at night.
Age and Growth
Life span is 7 years with maturity attained at 2-3 years.
Food
Scavenging for crustaceans, insects, molluscs, worms and less frequently macrophytes and fishes takes place at night. Older fish specialise on crayfish and fish in Lake Opinicon, Ontario. Young fish travel in large schools feeding generally on whatever is available.
Reproduction
Spawning occurs in May to July after both sexes construct a nest. The nest is an open depression on a stream bed, a short burrow in a stream bank or under a rock or log, or a deserted muskrat burrow. Eggs are cream, adhesive, up to 3.0 mm in diameter and laid in batches of up to 700. A female may contain up to 7000 eggs. The male guards the nest and the young until they reach 51 mm.
Importance
The flesh of this species is white and tasty and it is caught occasionally by anglers using baited hooks and incidentally in commercial fisheries.
Brown Bullhead / Barbotte brune
Ameiurus nebulosus (Lesueur, 1819)
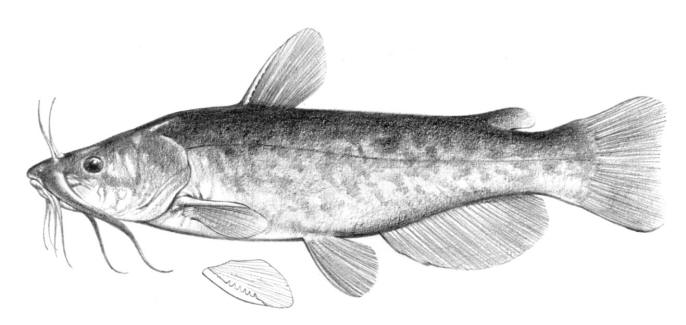



Taxonomy
Other common names include Northern Brown Bullhead, Mudcat, Mudpout, Horned Pout, Brown Catfish, Marbled Bullhead, Common Bullhead, Speckled Cat, Bullpout, Minister, Mud Cat, Creek Cat, Red Cat, Wooly Cat, Schuylkill Cat, Sacramento Cat. Formerly in the genus Ictalurus.
Key Characters
This species is identified by the posterior tip of the adipose fin being free of the back, the caudal fin is not deeply forked, mouth corner barbels are about twice as long as nostril barbels, there is no bony ridge between the head and the dorsal fin, the anal fin does not touch the caudal fin when pressed to the body, all barbels are dark brown to black, pectoral spine teeth strongly developed and numbering 4-9, total anal rays usually 20-24, gill rakers 10-16, caudal fin base dusky to dark, the dorsal fin membranes are not particularly dark, and the caudal fin is truncate.
Description
Dorsal fin soft rays 6-7, and pelvic rays 8. The dorsal fin spine has weak to absent teeth on barbs. Occasionally deformed specimens have been caught in the Ottawa River (Coad et al., 1974). These include s-shaped vertebral columns and major fusion of vertebrae resulting in a markedly shortened body. The causes are unknown.
Colour
The head, back and upper flanks are blue-black to yellow-brown or olive. There may be a violet iridescence. The flanks are lighter and may be mottled brown. The belly is yellowish to white. Barbels are dark brown to black. Fins are similar in colour to the adjacent body with some membranes darker. Faber (1984c) illustrates a larva.
Size
Reaches 62.0 cm and 3.35 kg, possibly 5.01 kg. The world, all-tackle angling record weighed 2.49 kg and was caught in 1975 in Veal Pond, Georgia.
Found in southern Nova Scotia, New Brunswick and Ontario, the extreme south of Québec, Lake of the Woods, southern Manitoba and southeastern Saskatchewan. Introduced to British Columbia in the lower Fraser River and southern Vancouver Island. In the U.S.A. south to Florida and Alabama. Widely introduced elsewhere in North America and Eurasia.
Origin
This species entered the NCR from a Mississippian, Missourian or an Atlantic coastal refugium. The presence of this species in Gatineau Park may be from introductions (Rubec, 1975a).
Habitat
This nocturnal bullhead lives in the shallow, weedy, warm waters of ponds, bays in lakes and river backwaters. Fish usually stay in a limited area but may migrate 16.1 km downstream in the Ottawa River. Bottoms are usually sand or mud with sunken logs down to about 12 m and this fish may burrow into mud to escape poor environmental conditions. A fish kill involving this species was noted at Mud Lake, Britannia Woods on 12 April 1984 probably from oxygen depletion under ice (Halliday, 1985). However it is tolerant of pollution, low oxygen and high temperatures (over 36°C, preferred temperature is 24.9-27.3°C). Rubec and Qadri (1982) report it as more abundant below industrial and municipal effluents in the Ottawa River near Ottawa. These effluents create turbid conditions that perhaps favour the touch-sensitive bullheads over visual feeders. However fish were in poorer condition and had slower growth where wood fibres from paper mills cover the bottom in the Ottawa River. Wood fibres limit invertebrate production and thus the areas suitable for bullheads to feed in. Numbers of bullheads can be high and below Ottawa they comprise 86% of the fish catch by number and 68% of the biomass with concentrations as high as an estimated 7785 fish per hectare in a bay on Kettle Island. Trap-nets set at the mouth of the bay in the early 1970s caught so many bullheads that they could not be hauled in and the net had to be cut to allow fish to escape. The construction of the Carillon Dam backed up water as far as Ottawa, flooding large areas and creating marshes ideal for bullheads below Ottawa. Bullheads in Lac LaPêche in Gatineau Park had markedly less pollutants (PCBs) in white muscle than fish from the St. Lawrence River east of Cornwall (Otto and Moon, 1996). Aggregations of bullheads have been seen prior to spawning, one during early May near Rockland in the Ottawa River being 45 by 27 feet in size. Some bullhead were observed to jump 2-3 feet out on the water (Rubec, 1975b). In the Spencerville Reach of the upper South Nation River, this bullhead dominated the hoop-net catch at 59% of the total catch (Lauzon, 2003).
Age and Growth
Maturity is attained mostly at age 3 and about 20-33 cm and life span is 18 years. Around Ottawa in the Ottawa River fish reach 9 years and populations had both fast- and slow-growing individuals (Rubec and Qadri, 1982). Some females mature as early as age 2. Males grow faster than females near Montréal and the population there includes both a slower and a more rapidly growing group. Stunted populations occur in poor environments where food is lacking and temperatures are low.
Food
Food is taken mostly at night aided by the sensitive barbels in an opportunistic manner. Gunn (1976) found that these catfish fed heavily in the early morning hours in the Ottawa River. Food includes crustaceans, insects, worms, molluscs, and less frequently algae and fishes. In the Ottawa River algae often comprise more than 60% of the diet, especially in summer, and include Spirogyra, Ulothrix, Oscillatoria and Fragilaria species. Experimental studies have shown about 24-67% of the carbon in the algae is digested as food and is an important energy source (Gunn, 1976; Gunn et al., 1977). Bullheads may eat duckweed, gulping this plant at the water surface in contrast to their normal bottom feeding. Bullheads also scavenge for waste and dead organisms and eat the eggs of commercially important species such as Lake Trout, although their impact on trout populations is probably not highly significant. Brown Bullheads are known to eat Pumpkinseeds. Walleye, Sauger, Northern Pike and Yellow Perch eat bullheads.
Reproduction
Spawning occurs in May to July in Canada at a water temperature over 20°C. In the NCR spawning occurs in about the third week of June at depths of 6 inches (15.2 cm) to 4 feet (1.22 m). A dominant male establishes a territory and prior to spawning fish caught in the Ottawa River show whitish scratches and teeth marks (Rubec, 1975b). The male and/or female, usually the latter, excavates a nest in mud, sand, among plant roots or in a burrow in a bank. Pebbles may be carried away in the mouth. The spawning pair touch and caress each others barbels and come to lie head to tail. Pale cream, adhesive eggs are shed and fertilised at intervals. Up to 13,800 eggs are recorded from 1 female, with fecundity in the Ottawa River reaching 8823 eggs (Rubec, 1975b). Both parents guard, fan and manipulate the ball of eggs, although Rubec (1975b) observed only the male doing this. Manipulation includes stirring with the barbels or fin spines and even picking up with the mouth and spitting out. Manipulation is essential for hatching. Larvae are 6.0-8.0 mm long at hatching. The young bullheads travel in a school for several weeks until they are about 5 cm long when they disperse. Either or both parents guard the school. These schools or catfish balls can number in the many hundreds and a ball can by walked through, parting and reforming behind one.
Importance
The flesh is red to pink and is excellent eating. Bullheads are easy to catch on worms, minnows, shrimps and cut meat. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
In the U.S.A. they are used in pond culture. There are some minor commercial catches in Canada, mostly in Ontario. Dymond (1939) records catches from 1896 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates and, in addition, includes some Channel Catfish. For example, in 1898 the catch was 55,850 lbs (25,356 kg) in the Ottawa River from Carillon to Pontiac in Québec, the highest recorded. In Ontario, the highest catch was in 1898 from Prescott and Carleton counties at 41,100 lbs (18,659 kg). McAllister and Coad (1975) report catches of about 100,000 lbs (45,500 kg) from the Ottawa River annually by commercial fishermen. Commercial fisheries for bullheads above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b). They occasionally appear in supermarkets in Ottawa as mudpout or barbotte. Barbotte suppers are held in the NCR by charitable organisations to raise funds. In 2005, the 5th Annual Pout Masters Catfish Tournament was held in Russell, Ontario in mid-May to raise funds for the Canadian Cancer Society. The Brown Bullhead has been used widely as an experimental animal to study uptake of pollutants and in physiological studies. Its introduction to waters where it is not native has severely affected other fishes through predation and competition.
Channel Catfish / Barbue de rivière
Ictalurus punctatus (Rafinesque, 1818)
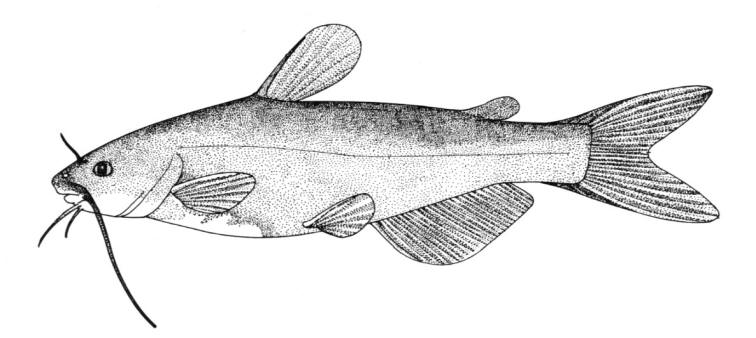

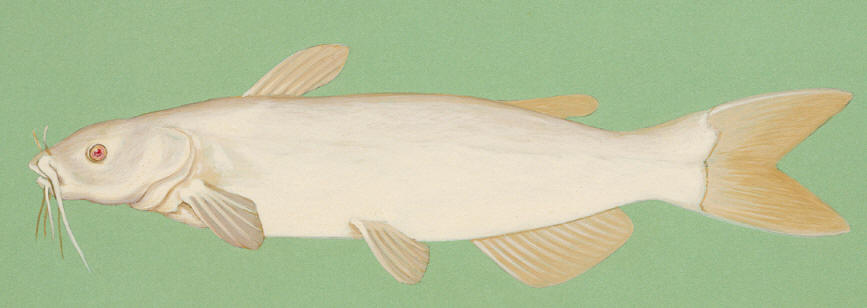
Taxonomy
Other common names include Spotted Catfish, Great Lake Catfish, Lake Catfish, Northern Catfish, Blue Channel Cat, Lady Cat, White Cat, Fiddler, Willow Cat, Blue Fulton, Chucklehead and Hirondelle.
Key Characters
This species is identified by the posterior tip of the adipose fin being free of the back, a characteristic deeply forked caudal fin, mouth corner barbels more than 3 times as long as nostril barbels, and a bony ridge between the back of the head and the dorsal fin.
Description
Dorsal fin soft rays 6-7, total anal rays 23-32, pelvic rays 8 and pectoral rays 8-9. The dorsal fin spine lacks barbs while the pectoral spine has 16-20 strong barbs posteriorly. Gill rakers 13-18.
Colour
The back and upper flank are steel-blue, blue-grey, slate-brown or grey, fading to a whitish belly. Fins are similar to the adjacent body. Barbels are blackish. Males develop a swollen head above and behind the eyes in the spawning season, the bluish back colour is brighter and the belly is whitish-blue. Young fish, smaller than 35.6 cm, have olive to blackish dots on the flank and are greenish or silvery. An albino catfish, possibly a Channel Catfish, was caught on a worm in the Ottawa River at Treadwell just east of the NCR on the evening of 12 July 1999 and was returned there a few days later (N. Villeneuve, personal communication, 1999; Aubry, 1999). A "white catfish" was also caught in Constance Bay within the NCR in the early 1960s.
Size
Reaches 120.2 cm, possibly 150 cm, and more than 50.0 kg. The world, all-tackle angling record weighed 26.3 kg and was caught in 1964 in Santee-Cooper Reservoir, South Carolina. A 20.2 kg fish is reported from the Red River, Manitoba. Small (1883) found fish of 10 lbs (4.5 kg) and over only occasionally on the market in Ottawa. A Channel Catfish of 14 lbs (6.4 kg) was reported from the Ottawa River in April 2002 (www.fish-hawk.net, downloaded 20 May 2003) and fish to 40 lbs are reported from below the hydro dam at Fitzroy Harbour on the Ottawa River (www.neatpage.com/species.htm, downloaded 3 June 2003). The average size of Ottawa Valley "catfish", presumably this species, is given as 9 kg (www.ottawavalley.org/trivia/triv_pg.html, a cache downloaded 13 June 2003) while the Lac des Chats is reported as having fish in the 0.9-1.8 kg range with some larger fish at over 4.5 kg ( www.fishontario.com/articles/channel-cats/ontario.html, downloaded 20 June 2003). Smith (1974) found fish in the Ottawa River to reach 66.6 cm fork length.
Found in extreme southern Québec, southern Ontario, Lake of the Woods, southeastern Manitoba and in the Qu'Appelle River and Cumberland Lake in eastern Saskatchewan. South to the Gulf of Mexico in the U.S.A. Widely introduced outside its natural distribution. In the major rivers of the NCR including the South Nation River (www.fish-hawk.net/gallery/misc/misc.htm?fuseaction=home.models&nav=4, downloaded 19 June 2003). More common above Ottawa-Hull than below.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Channel cats are found in lakes and the larger rivers favouring deeper, cooler and clearer water than other bullheads. However they can survive temperatures up to 35°C although preferred temperature is 25.2°C. They are found as deep as 60 feet (18.3 m) in the Ottawa River (McAllister and Coad, 1975) over sand and rock bottoms. Channel Catfish were the predominant species in a nearshore community index netting of Lac des Chats (a section of the Ottawa River), making up 65.6% of fish sampled (Haxton, 1999). They usually spend the day in deep holes or under rocks and logs. In the St. Lawrence River some channel catfish do not move while others travel as much as 160 km and in the Red River in Manitoba as much as 350 km in 13 days. One fish tagged in the Ottawa River was caught 4 months later 16 km upriver (Smith, 1974). This fish is relatively absent downstream from Ottawa-Gatineau compared to upstream although 50 km downriver from the capital it is abundant enough to be commercially important. No clear reasons was discerned but pollution, food availability and competition with Ameirus nebulosus are possibilities (Smith, 1974). In Lac Deschênes of the Ottawa River, Haxton and Punt (2004) found densities of 3.5-6.4 fish/ha with estimated population size there at 38,445-69,609 fish. In Lac des Chats, which lies mostly outside the NCR upriver from Lac Deschênes, density was 29.4 fish/ha and abundance was 220,924 fish.
Age and Growth
Life span is about 40 years although few fish attain this. Maximum age in the NCR is 18 years but most are less than 13 years old (Smith, 1974; McAllister and Coad, 1975). Haxton and Punt (2004) aged fish to 26 years in the Ottawa River but this included fish from outside the NCR. Smith (1974) found fish in the Ottawa River showed similar growth for each sex, in both length and weight. All females were mature by age 6, before 33 cm fork length and 50% were mature at 5 years while all males were mature by age 11, before 45 cm fork length and 50% were mature at 6 years. This is older than in southern latitudes although the rate of increase in length was similar to other Canadian and American populations, even more southerly ones (Smith, 1974). A sample of 832 fish from Lac des Chats (Ottawa River section) had a mean total length of 47.4 cm and a weight of 1132 g, with a mean maximum length of 74.5 cm and a mean maximum weight of 5000 g (Haxton, 1999). These fish matured at total lengths over 40 cm, the smallest mature male being 42.1 cm and the smallest female 44.0 cm. Fish from Lac Deschênes had a mean length of 51.5 cm and a mean weight of 1539 g in one sample period, 48.5 cm and 1298 g in another (Haxton and Punt (2004). In Lake Erie half the females are mature at 25-28 cm and half the males at 28-31 cm. Growth is slower in the Ottawa River compared with other populations (Haxton and Punt (2004), probably due to very sporadic production of year classes and greatly reduced growth of older fish. Growth after 8 years of age is slow in Québec waters and maturity is attained there at 8 years at the northern range limit.
Catfish can be aged by the growth rings on spines which also reflect water temperatures since catfish do not feed and grow below certain temperatures. This knowledge gained from extant catfish has been used to determine the climate millions of years ago from deposits of fossil catfish spines.
Food
Feeding occurs both by day and by night and some items are taken at the water surface. Food includes crustaceans, aquatic and terrestrial insects, molluscs, algae and other plants, seeds, fish such as Yellow Perch, Pumpkinseeds, Mooneyes, Yellow Walleye, darters and minnows, and even birds, grapes, chicken necks, canned corn, beef bones and other scavenged items. In the Ottawa River, Smith (1974) found fish up to 30 cm fork length were insectivorous, from 30-45 cm omnivorous and above 45 cm piscivorous. The food consumed varied with seasonal availability and was taken mostly in aquatic vegetation. Channel cats elsewhere larger than about 80 cm feed almost exclusively on other fishes. Food in the Lac des Chats catfish included crayfish, insects, plants, juvenile Northern Pike, Walleye, Pumpkinseed and Smallmouth Bass (Haxton, 1999).
Reproduction
Spawning occurs in late spring to summer at 21-30°C; in the Ottawa River from late June to early July at 20.6-23.3°C (Smith, 1974). Some channel catfish move into running water at spawning. The male builds and cleans a nest, using fin and body movements, in holes under rocks, logs or in banks. Females bite other females or males when ready to spawn and both sexes drive other fish away. The female moves over the nest site, wiggling and banging the pectoral and pelvic alternately on the bottom. The male and female position themselves head to tail, wrapping their tails over each other's head, both fish quiver and release eggs and sperm. Eggs are yellow, up to 4.0 mm in diameter and up to 52,000 per female. In the Ottawa River average fecundity is 6480 eggs and average diameter 3.6 mm at spawning with maximum fecundity 11,500 eggs and diameter 4.18 mm (Smith, 1974). Males defend, aerate and manipulate the eggs. Manipulation is carried out with the body and fins and may serve to aid in hatching. The young fish are not herded as in bullheads.
Importance
Channel catfish have white, flaky flesh which has attracted a commercial fishery in Canada, and it is the principle catfish on farms in the U.S.A. in an attempt to meet demand. It is said to be one of the best tasting freshwater fishes in North America. The Canadian catch reached 564,322 kg in 1964. Dymond (1939) records catches from 1903, 1904 and 1907 in the NCR from the Ottawa River and tributaries (Pontiac and Ottawa counties) with weights of 11,600, 9000 and 8100 lbs (5266, 4086 and 3677 kg) respectively. McAllister and Coad (1975) report a commercial catch of 1000-2500 lbs (454-1135 kg) in the Ottawa River annually. Commercial fisheries for catfish above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b).
A 40 km stretch of the Ottawa River near Arnprior (Lac des Chats) was estimated to hold 252,350 fish (Haxton, 1999; Egan, 2002). The "Ottawa River Catfest" started in the year 2000 based in Arnprior and in 2002 over 3000 fish were caught by 500 anglers in the 3-day event. Fishing pressure in low in the Ottawa and the annual mortality of 15.7% is probably natural mortality (Haxton and Punt, 2004). This species is not an important sport fish in Ontario, despite "Catfest" and the Ottawa River population is underutilised (Haxton and Punt, 2004).
This catfish is an important sport species taken with live or dead bait, preferring the latter. It has even been caught using white soap as bait. Their large size, fighting abilities and tastiness make them attractive to anglers. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
A fish kill, predominately of this species, in the Ottawa River, mostly above the Chats Falls Dam near Arnprior but also down to below Rockland (on a map, elsewhere to Petrie Island "near the town of Rockland"), took place in August 2006. It was attributed to an outbreak of columnaris disease (Ottawa Citizen, 20 August 2006, p. A3; Punt and Castro, 2006). The disease manifests as brown to yellow sores on the skin, fins and gills. Initially skin sores, for example, are very shallow and just appear as skin areas lacking the natural shiny appearance. Later this develops into round to oval lesions with an open ulcer in the centre. A characteristic lesion encircles the body as a pale white band, called a saddleback. Between 2000 and 4000 fish were killed. The disease is caused by Flavobacterium columnare, usually occurs in summer and is associated with stress, crowding, injury and poor water quality. In this case, high temperatures, torrential rain and extreme runoff events in late July and early August caused warm temperatures in the river and a stressed environment.
Stonecat / Barbotte des rapides
Noturus flavus Rafinesque, 1818
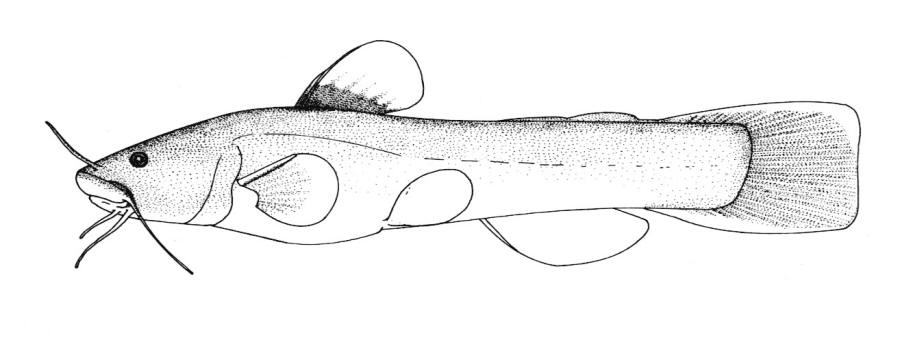

Taxonomy
Other common names include Stone Catfish, White Cat, Doogler, Beetle-eye, Mongrel Bullhead, Deepwater Bullhead and Little Yellow Cat.
Key Characters
This species is identified by the posterior tip of the adipose fin not being free but attached to the back, pectoral spine nearly straight to moderately curved, with or without anterior teeth, posterior edge smooth to roughened, and premaxillary band of teeth with lateral processes extending backwards.
Description
Dorsal fin soft rays 5-7, usually 6, anal rays 15-19, pelvic rays 8-10, usually 8, and pectoral soft rays 8-11, usually 10. There are 2 internasal pores.
Colour
The head, back and upper flank are dark bluish-olive, blue-black, olive, slate-grey or yellow-brown fading to cream, white or grey on the belly. The dorsal fin has a grey blotch at its base. The adipose fin is dark with a pale edge. Fin margins are light to white. There is a light yellow spot behind the dorsal fin across the back and a light blotch on the nape. The caudal fin has a white blotch on its upper margin. Chin barbels are white and upper barbels grey.
Size
Reaches 31.2 cm and 0.482 kg.
Found in the Mississippi and Hudson river basins and the Great Lakes and Hudson Bay basins. In Canada in the southern Ontario basins of lakes Huron, Erie and Ontario and the upper St. Lawrence River basin of Ontario and Québec. It just enters Saskatchewan and Alberta in the Missouri River basin and in Manitoba is in the Assiniboine and Red River basins. A record of this species from the Ottawa River by Halkett (1913a) was based on "A very small specimen some 1¾ inches long passed from the Ottawa River through the water taps of the Ottawa fish hatchery in February, 1909". Boucher (2005) reports it from the Ruisseau La Pêche and the Rivière de la Petite Nation in the Outaouais (not on the map here).
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
The favoured habitat of this species is riffles of streams and lake margins with water movement by wave action. It usually lives in the spaces between stones but may be found in stream mouths over mud and sand bottoms. It tends to be a solitary species. Boucher (2005) summarises biology of this species.
Age and Growth
They may live 10 years. Females are mature at 4-5 years of age and males at 3 years.
Food
Food is mainly larval insects such as mayflies, and crustaceans, particularly crayfish, with molluscs, small fishes and algae. Feeding probably takes place at night. An aquarium specimen from the NCR fed on small guppies at night making a sharp clicking sound as it seized its prey (McAllister and Coad, 1975). Snakes and Smallmouth Bass are known to be successful predators despite the stinging pectoral fin spine.
Reproduction
Spawning occurs in summer (June to August) at high water temperatures (usually 27-29°C, but sometimes as low as 23°C in Manitoba). The male excavates a nest under a stone or under refuse such as cans, bottles and boxes. A sticky mass of about 100-500 eggs is laid under a stone and guarded by the male. Each female can produce up to 1205 yellow eggs of up to 4.0 mm diameter.
Importance
Stonecats have been used as bait for bass and catfishes. Their spines are venomous, painful but not fatal to humans. They are said to be quite tasty though rather small to eat.
Tadpole Madtom / Chat-fou brun
Noturus gyrinus (Mitchill, 1817)
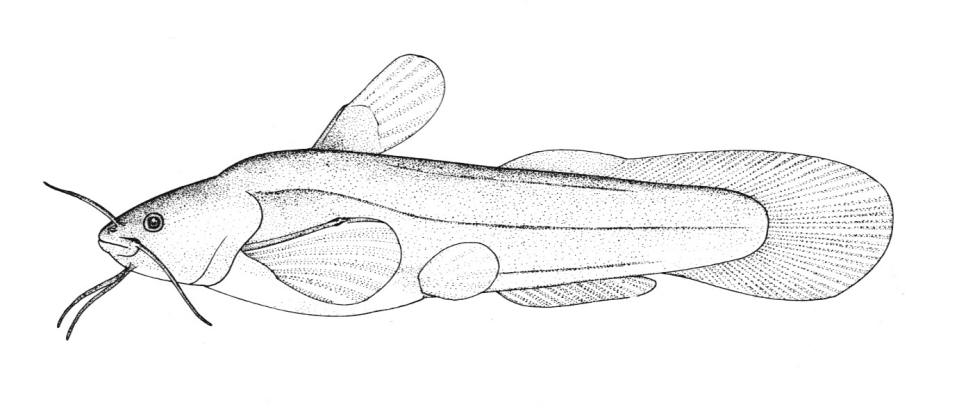

Taxonomy
Other common names include Tadpole Stonecat and Tadpole Cat.
Key Characters
This species is identified by the posterior tip of the adipose fin not being free but attached to the back, pectoral spine nearly straight to moderately curved and without teeth, premaxillary band of teeth without lateral processes extending backwards, pectoral soft rays usually 7, but a range of 5-10, pelvic rays usually 8 but range of 5-10, mouth terminal, typically 10 preoperculomandibular pores, and vertical fins without a black margin.
Description
Dorsal fin soft rays 4-7, usually 6, anal rays 12-18, usually 15. There are 2 internasal pores. Body shape resembles a tadpole, hence the name. The anterior rays of the caudal fin are more strongly developed than in any other madtom.
Colour
The back and upper flank are olive-grey or brown to golden yellow, with the lower flank and belly light or with some pigmentation. Fins and barbels may be either darker or lighter than the adjacent body. The muscle segments of the body are darkly outlined and there is a dark line along the body axis. Breeding males are a dark chocolate brown or brownish-dusky.
Size
Attains 11.5 cm.
Found in Atlantic coast drainages from New York to Florida, along the Gulf coast, in the Mississippi River basin and the southern Great Lakes basin. Absent from the mountains paralleling the Atlantic coast. In Canada found in southern Saskatchewan and Manitoba, the Quetico-Rainy River drainages of western Ontario which drain to Hudson Bay, tributaries of Lakes Huron, St. Clair, Erie and Ontario, and upper tributaries of the St. Lawrence River in Ontario and Quebec.
Origin
This species entered the NCR from possibly an Atlantic coastal or Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
This madtom is found in quieter waters with slow current over mud bottoms with lots of vegetation such as wild celery and cattail beds in the Rideau River (Phelps et al., 2000). It may be found in both streams and lakes down to 25 m. It is nocturnal and hides in holes, debris or vegetation during the day but is quite common.
Age and Growth
Maximum age is 4 years. Males are larger and heavier than females in their fourth year of life. Some fish mature as early as 1 year and most by 2 years of age.
Food
Food is various aquatic insects and crustaceans. The toxin in the pectoral spine is capable of immobilising a Northern Pike.
Reproduction
Spawning occurs in summer (probably July in Canada) and eggs are laid in cavities or even tin cans. F. W. Schueler (in litt., 11 August 2004) found a ca. 70 mm adult in a 5 cm, roughly spherical nest under a stone with a carpet of newly hatched young on 8 July 1987 in Kemptville Creek just northwest of Oxford Mills at a water temperature of ca. 27ºC. Attempts to catch more advanced young in another nest were unsuccessful as they disappeared into the gravel. Eggs are yellow, up to 3.5 mm in diameter, but very variable in size, and are in an adhesive mass surrounded by a gelatinous envelope. Up to 150 eggs are found together, perhaps from more than 1 female. Clutches may be guarded by both male and female, or the male alone. Each female may contain up to 323 eggs but fecundity, like egg diameter, varies between populations.
Importance
This madtom has been used as bait for other fishes by anglers and is said to be excellent, despite the venomous pectoral spine.
Margined Madtom / Chat-fou liséré
Noturus insignis (Richardson, 1836)
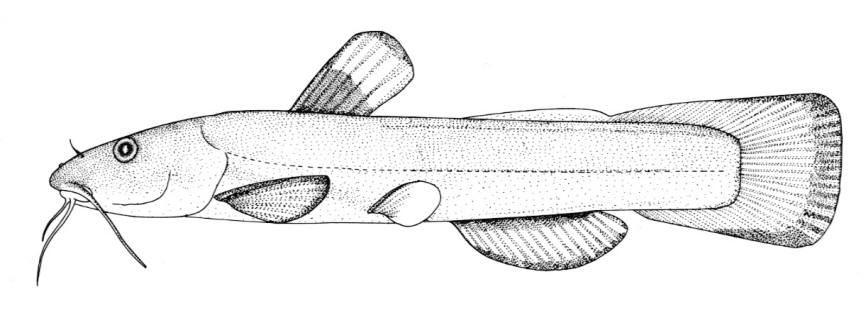

Taxonomy
Another common name is Chat-fou livré.
Key Characters
This species is identified by the posterior tip of the adipose fin not being free but attached to the back, pectoral spine nearly straight to moderately curved, without anterior teeth, posterior teeth present, premaxillary band of teeth without lateral processes extending backwards, pectoral rays usually 9, pelvic rays usually 9, lower jaw included, 11 preoperculomandibular pores, and particularly the vertical fins with a black margin.
Description
Dorsal fin soft rays are 5-7, usually 6, anal rays number 15-21. Pectoral ray range is 7-10, pelvic ray range 8-10.
Colour
Overall colour is olive, slate-grey or yellowish fading to cream on the belly. Chin barbels are creamy white and other barbels dark. A narrow dark band crosses the belly in front of the pelvic fins. The dark band just below the edge of the dorsal, anal and caudal fins give this species its name. The extreme edge of these fins is clear. The pelvic fins are clear and the pectorals have a marginal band.
Size
Reaches 17.9 cm total length.
Found in Atlantic coastal streams from New York south to Georgia, upper Ohio, Tennessee and Kanawha tributaries, southern tributaries of Lake Ontario. In eastern Ontario known from the Fall and Mississippi rivers and Bolton Creek in Lanark County near the NCR. It was first recorded from Canada in Gatineau Park in a stream draining Lac à la Loutre to Lac Lapêche (Rubec and Coad, 1974) and is now known from connected waterways in the Park and in the Gatineau River (www.fapaq.gouv.qc.ca, downloaded 7 October 2002). Also recorded between Hull and Lochaber, Hull County and between Lochaber and Montebello, Papineau County on the Québec side of the Ottawa River, and from the Cole Lake area near Buckingham (Phelps and Francis, 2002). In July 2005, this species was collected from the Mississippi River at Carleton Place and Appleton.
Origin
This species is used as a hardy bait fish in the U.S.A. so its recently recorded presence in Canada and the NCR may be the result of live release of specimens carried into Canada (Rubec and Coad, 1974; Coad, 1986a). There are documented records of bait fish introductions surviving in U.S.A. streams and so this is a plausible explanation for their appearance in Canada, especially since the Canadian localities are in popular areas for sport fishing. But it should be noted that there are restrictions on use of live bait fish in Ontario and Québec, although whether these would be followed in all cases by visitors from the south is debatable. Emmett and Cochran (2010) describe how Largemouth Bass will take another species of madtom (Noturus gyrinus) without avoidance or apparent long-term effects, so their use as bait is not as implausible as it might seem.
Alternatively the species could have an origin from an Atlantic refugium after the last glaciation when a warmer climate existed than does today. Interconnecting postglacial lakes could have facilitated dispersal. It may also be a recent immigrant assisted by canals in the U.S.A. which have been shown to affect distributions. However the waterfall at Wakefield, Québec has been an effective recent barrier to dispersal into Gatineau Park for various fish species. Goodchild (1990a) reviews these possibilities and tentatively concludes that the species is indigenous to Canada and the NCR. The continued discovery of new localities for this species in the NCR suggests that it is spreading, which can argue for an introduction at one site in Gatineau Park, Québec and one on the Mississippi River in Ontario, followed by dispersion. Alternatively, the initial discovery can be seen as stimulating active searches for this species using appropriate equipment such as back-pack electroshockers, revealing distributions that existed before but were not apparent before. DNA studies currently under way may resolve the issue.
Habitat
It prefers clear streams with riffles of gravel and stones and in some cases with rocks and boulders and disappears when these silt up, e.g. after beavers dammed streams in Gatineau Park (Coad, 1986a). It is also recorded from a rubble shoal in Lake Joseph, Québec outside the NCR. This madtom is secretive and nocturnal. Numbers may be small at any one locality and this species can be easily over collected, removing the population at that locality. Coad (1986a) failed to find them using a seining technique that had been successful before but later, using an electroshocker was able to capture them in neighbouring localities in the same drainage. Lacoursière et al. (1989) failed to find any in Gatineau Park in their 1988 field study but their specimens were not seen by me. Wet specimens can survive several hours in air and this contributes to its use as a bait fish. However they are susceptible to the low oxygen concentrations found in late summer in pool areas of streams (Goodchild, 1990a). Laboratory hatching of eggs was optimal at 28-30ºC, and this may restrict distribution.
Age and Growth
Females are mature at 2 years, males a little earlier. Maximum age is 5 years. Most growth occurs in the summer after spawning.
Food
Food is insects, particularly those most active between midnight and dawn, crustaceans and, less frequently, fish, usually taken at night.
Reproduction
Adhesive eggs are deposited in a mass in a nesting cavity and are placed such that a current of water brings oxygen to them. The male cares for the eggs but may abandon or eat them if disturbed. Spawning occurs in June-July and females have up to 223 orange eggs with a diameter of 4.0 mm.
Importance
It was designated as "Threatened" in 1989 by the Committee on the Status of Endangered Wildlife in Canada (Goodchild, 1990a) but placed in the "Data Deficient" category because of insufficient scientific information in 2002. Lemieux (1986) recommended that it be given special attention in ecological analyses of Gatineau Park because of its rarity and apparent decline and Lacoursière et al. (1989) found none despite a specific effort to locate specimens. The fin spines are venomous but not fatal to humans.
The pikes, pickerels and muskellunge are found in fresh waters of the Northern Hemisphere. They are moderate to large-sized fishes. There are only 5 species and 4 of these occur in Canada with 2 in the NCR.
The family is characterised by a flattened, elongate, duck-billed snout. Teeth are present on the tongue, teeth on the basibranchial bones behind the tongue are small, jaws have large teeth, branchiostegal rays number 10-20, the swimbladder is connected to the gut by a duct, intermuscular bones are forked or y-shaped, there are no fin spines, pelvic fins are abdominal, cycloid scales are present, gill rakers are present as sharp denticles in patches, there are no pyloric caeca, the lateral line is complete, and the forked caudal fin has mostly 17 branched rays.
Pikes are related to the Mudminnow Family by such characters as no adipose fin, dorsal and anal fins far back on the body near the tail, the upper jaw is bordered by a toothless maxilla, and pyloric caeca and the mesocoracoid bone in the pectoral girdle are absent. "Pike" are named for their pointed snout and "pickerel" is a Middle English word for a small pike.
Pikes are predators on other fishes aided by the posterior dorsal and anal fins which facilitate rapid darts forward. They are important sport fishes, much sought after by anglers for their fighting ability, but are not very good eating because of the intermuscular bones.
Northern Pike / Grand brochet
Esox lucius Linnaeus, 1758
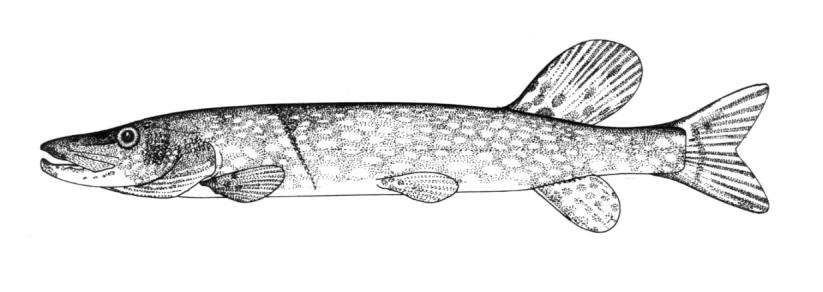


Taxonomy
Other common names include Pike, Great Northern Pike, Pickerel, Snake, Wolf, Gator, Slinker, Great Lakes Pike, Canada Pike, Shovelnose Pike, Channel Pickerel, Short Pickerel, Marsh Pickerel, Hammer Handle, Slough Shark, Snakefish, Grass Pike, or Jack (young Pike), Brochet commun, Grand brochet du nord, Sjulik, Ihok, Siun, Hiulik, Siolik, Kikiyuk, Idlûlukak. A pike and Muskie hybrid (a "Tiger Musky") is reported from the Ottawa Reach (from Hog's Back downriver) of the Rideau River (Wachelka et al., 2000; RMOC, 2000; Setterington, 2004) although it is not clear if this was the same fish in all reports.
Key Characters
This species is distinguished by having 9-11 pores on the lower jaws (usually 5 on each jaw; the Muskellunge has higher counts), the cheeks are fully scaled, and the overall colour is dark with light spots, the reverse of that in Muskellunge.
Description
Dorsal fin principal rays 15-19, principal anal rays 12-16, pectoral rays 13-17 and pelvic rays 10-11. Lateral line scales 105-148.
Colour
The back and upper flank are dark green, olive-green or brownish, fading to a whitish belly. The flank has 7-9 rows of greenish, yellow to whitish blotches along it. Scales have a golden tip. The head sides have wavy, golden or yellow blotches and lines and the eyes are bright yellow to golden. The dorsal, anal and caudal fins are green, yellow, orange or pale red, blotched and barred irregularly with black. The pectoral and pelvic fins are dusky to orange. Young have 8-15, wavy, white or yellow bars which become the bean-shaped blotches in adults as they gradually break up. There is a gold to green stripe along the middle of the back in some fish but others are completely dark green. There is a stripe below the eye in young less than 4 cm long. Adults have a vertical bar below the eye. A variety, known as Silver Pike, has an overall silvery colour without flank spots. Faber (1984c) illustrates a larva.
Size
Reaches 1.75 m total length and 48.0 kg, possibly to 2.13 m and 65.8 kg. The world, all-tackle angling record weighed 25 kg and was caught in Germany in 1986. The Ontario record as of the year 2002 weighed 19.1 kg and was from Delany Lake near Kenora. A pike of 20¼ lbs (9.2 kg) and 44¾ inches (1.14 m) length was caught in Boman (= ?Bowman) Lake in the NCR in 1961 (Anonymous, 1961b), a 20 lbs (9.1 kg) fish was taken in Pontiac Bay. (www.neatpage.com/fishing/species.htm, downloaded 20 May 2003), a 25 lbs fish was taken in Lac Lapêche (local informant to A. Martel, 2003), and a 18lbs one from Constance Lake (www.constancelake.com/articles.htm, downloaded 26 April 2004).
Found from Labrador and Québec (but not the Maritimes and Gaspé) west to Alberta, northern and northeastern British Columbia, Yukon, mainland N.W.T. and Alaska. In the U.S.A. south to Missouri east of the Appalachian Mountains. Also across northern Eurasia. This species has been stocked in the Rideau River in the early 1940s (Phelps,2001).
Origin
This species entered the NCR from a Mississippian refugium, or possibly from a Beringian or Missourian refugium (Mandrak and Crossman, 1992).
Habitat
Pike are solitary and are found in lakes and rivers where the water is still or flowing slowly. Vegetation is moderate to heavy and cover such as fallen trees and boulders is available. Shallow marshy backwaters or the floodplains of rivers are required for spawning. Relatively good water clarity is required for detecting prey (>1-2 m). They prefer warm water with 20-25° optimal for growth and 6-12°C for spawning, but they usually retire to deeper, cooler water at the height of summer. They are tolerant of high temperatures up to 32°C and low oxygen but prefer >4 mgL-1. pH 5.0-9.0 is tolerated. Pike are active in winter as anglers can testify. A fish kill along extensive stretches of the Rideau River and Canal and in Mackay Lake, Rockliffe was reported in spring 1956 (newspaper reports). Summer distribution is usually within 300 m of shore and less than 4 m deep. On windy days, Pike retreat offshore in surface waters. Large fish often occur in deeper water along rocky shores, down to 12 m. Small (1883) reports pike in the NCR as basking listlessly at the surface during summer in shallow water. Fry of other species swim around the pike and are not attacked. In the fall pike spend the day in deeper water but enter shallows at night, even to the extent that their fins are exposed. This may be to enjoy the warmer water there, to remove parasites by rubbing on gravel, or "for the purpose of watching for and capturing small land animals that come to the water's edge at night".
Age and Growth
Life span is up to 26 years but is less than half this in fast-growing southern populations in Canada compared to Arctic fish. Some aquarium fish have lived 75 years. Age groups up to 9 years are recorded for the Mississippi Lake and Mississippi River at Carleton Place (Kerr, 1999c) and in Lac McGregor in the Outaouais (Vallières and Fortin, 1988) while intrrduced fish in Ramsay Lake in Gatineau Park reached over 7 years (Vachon et al., 2006). Maturity, like growth, varies with latitude and habitat. Lac McGregor fish fare well compared with other southern Québec populations, reaching 6.3 kg at age 9. Males mature at 1-6 years and females at 2-6 years. Females grow larger, faster and live longer than males. Adults on the Lac McGregor spawning run averaged 59.0 cm and 1589 g for females and 53.9 cm and 1380 g for males (Chatelain, 1976). Growth is best at 19-21°C and is very efficient.
Food
Food is initially zooplankton and aquatic insects but fish begin to predominate at 5.0 cm after about 1 month's growth. Muskellunge are not usually found with pike as pike spawn earlier and the young pike will feed on Muskie fry. Insects, cladocerans and isopods dominate in young pike (20-95 mm long) in Lac McGregor in the Outaouais (Vallières and Fortin, 1988). Over 90% of the diet of adults is fish, but frogs, crayfish, mice, muskrats and ducklings are taken. The delta areas of the Saskatchewan and Athabasca rivers lose an estimated 1.5 million waterfowl to Pike each year, about one-tenth of the annual production. In Lac Ste. Anne, Alberta, Pike feed on Yellow Perch, Spottail Shiners, Burbot and White Suckers in order of importance. The daily ration was high from May to August with a June peak and very low in winter. Food is seized after a rapid dart from concealment. Cylindrical fish like Yellow Perch are preferred over more deep-bodied species like Bluegills as being easier to swallow. Adults are attacked by lampreys and on the spawning grounds by bears and large, predatory birds. Young are eaten by various other fishes (e.g. Rock Bass in Lac McGregor (Veilleux and Lafrance, 1970)), birds and even large aquatic insects - a small pike was caught in Lac McGregor in the jaws of a larval Dytiscus beetle (Vallières and Fortin, 1988). Both sexes fast during spawning but females have rations 1.5-2.3 times as much as males in summer and winter.
Reproduction
Spawning runs occur in the late evening and early night, several days before spawning occurs. Generally males precede females onto the spawning grounds although at Lac McGregor in the Outaouais the migrations are similar among the two sexes. Males outnumber females 1.19-2.79:1 on the spawning run in this lake. Males have a migration lasting 14-22 days in Lac McGregor and females 13-18 days with the time elapsed from beginning of the migration to the end of the return migration being 22-25 days (Vallières and Fortin, 1988). Spawning takes place during the day, often in mid-afternoon, in shallow bays or flooded fields just after ice melt in late March to May. The Outaouais region in general has start dates of 27 March to 9 April and end dates of 12 April to 20 May, the marsh at Thurso starts 14 April and ends 3 May and Lac McGregor has start dates of 1 April to 21 April and end dates of 15 April to 12 May (Breton, 1978; Vallières and Fortin, 1988). Peak spawning in the Carillon stretch of the Ottawa River near the eastern edge of the NCR peaked at 20-27 April (Hydro-Québec, 1996). Water temperatures on the spawning run are as low as 1.1°C. Spawning itself takes place at temperatures several degrees warmer than this, up to 17.2°C. At Thurso temperature is 4-15°C. The spawning period is about 10 days at any one site. Each female is accompanied by 1-2 males as she swims over vegetation in the shallow water. Both sexes roll to bring their genital regions close together, vibrate and release 5-60 eggs and the sperm. Tail sweeps scatter the eggs. This 3-10 second process is repeated many times each day. Eggs are amber, 3.2 mm in diameter, adhesive and each female can produce up to 595,000. Eggs hatch 12-14 days later and the fry attach to vegetation by an adhesive head gland until the yolk sac is absorbed 6-10 days later. Larvae are 7.0-9.0 mm long at hatching.
Importance
Pike form a significant part of the freshwater commercial catch in Canada, about 4100 tonnes in 1988 worth almost $3.3 million. They rank after Lake Whitefish, Lake Trout and Walleye in Saskatchewan. In Alberta Pike were used for mink food and are exported to French consumers.
Moving pike to another water body is illegal in Ontario as they are predators, e.g. as noted above they, will eat Muskie fry, and they can change the ecosystem dynamics. Vachon et al. (2006) describe how the introduction of pike into Ramsay lake in Gatineau Park caused the probable disappearance of three fish species, namely Margariscus margarita, Culaea inconstans and Gasterosteus aculeatus, with several others expected to disappear in the next few years.
Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1903 the catch was 95,630 lbs (43,416 kg) in the Ottawa River and tributaries (Québec), the highest recorded. The catch in Ontario in 1891 from the Ottawa River fronting on Prescott, Russell and Carleton counties and inland waters was 13,125 lbs (5959 kg). Commercial fisheries for pike above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b) for the period before 1964.
Pike may carry the cysts of Diphyllobothrium latum, the broad fish tapeworm, a cestode which can infect humans with the adult stage if incompletely cooked flesh is eaten. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
They are sold fresh and make excellent eating, having white, flaky flesh, perhaps not as good as Walleye or Salmon Family members. A muddy taste in summer is attributed to the skin mucus and can be avoided by removing the skin before cooking. Pike are good sport fish taken by trolling spoons, plugs, fishes and worms near or off weed beds. They may also be taken with minnows suspended below a float. Wire leaders are used because of the Pike's sharp teeth. They are also an important element of the winter ice fishery taken with live or salted fish as bait or by jigging. Size limits are imposed on anglers but research shows this must be done with care. A large limit might seem to protect young fish so more survive to reproduce, but often more large females are taken and egg production decreases. A careful balance must be struck. In 1999 it was the third most frequent species at 11.5% (after "basses" at 37.4% of events and Walleye at 16.0%) sought at competitive fishing events in Ontario (Kerr, 1999d). One of the more fascinating aspects of Pike lore is the stories which have accumulated over the centuries. The Emperor's Pike, 579.5 cm long and 250 kg, was purportedly caught in a lake near Württemburg in 1497 and found to have a copper ring around its gill region relating in Greek its release by the Emperor Frederick II on 5 October 1230. The skeleton of this monster was kept in Mannheim Cathedral but was shown to have extra vertebrae taken from several Pike. The story itself is untenable even without the physical disproof. The Pike would have had trouble growing with a copper ring around its gill region and a Pike 579.5 cm long should have weighed more like 1.5 tons. A general belief in giant Pike made stories of people being pulled into ponds, hands being severed, infants in the Pike stomach, and so on more acceptable. While these European tales can be discounted by the practical North American, one Pike myth current in Canada is that they lose their appetite and their large canine teeth in midsummer when one would expect them to be actively feeding and biting on anglers' lures and baits. Scientists regard this as an angler's excuse. Pike do lose teeth but replace them regularly and always have an efficient set. Pike don't bite well in August because prey is abundant and they are well fed, and they tend to retreat to cooler waters at this time.
Muskellunge / Maskinongé
Esox masquinongy Mitchill, 1824



Taxonomy
Other common names include Musky, Muskie, Lunge, Jack, Chatauqua; and Tiger, Spotted, Barred, Great, Leopard, Great Lakes, Ohio or Wisconsin Muskellunge, and Maskinonge. Muskellunge and Maskinongé have been variously attributed to Latin or Québecois for long mask (masca longa, masque allongé) and various Indian words. There is an unnamed smaller, lake form of this species in Ontario which is barred rather than spotted and has a different biology, but its presence in the NCR has not been determined (Hubbs et al., 2004).
Key Characters
This species is distinguished from Northern Pike by having a total of 12-20 pores on the lower jaws (usually 6-10 on each jaw), only the upper half of the cheek is fully scaled, and by colour pattern.
Description
Dorsal fin with 15-19 principal rays, anal fin with 14-16 principal rays, pectoral rays 14-19 and pelvic rays 11-12. Lateral line scales 130-176.
Colour
The bank and upper flank are golden green to brown, or yellowish, flanks similar or grey or silvery and the belly is whitish with small brown or grey spots and blotches. The head has spots on the sides or bars radiating from the eyes. Flanks have dark brown to black spots, bars, blotches or worm tracks (vermiculations) on a lighter background. The dark markings vary considerably between and within populations. Very large fish are more silvery and such markings are obscured. Fins are green to red-brown and the dorsal, anal and caudal fins in particular are blotched with dusky spots. Young have a gold or gold-green stripe down the middle of the back, upper flanks and back are blue-green to tan fading to white below and the flanks have irregular, dark blotches or oblique bars. Hybrids with Northern Pike are strongly barred and called Tiger Muskellunge.
Size
Reaches over 1.83 m and 45.0 kg but most are much smaller. Possibly to 2.44 m and 73.5 kg. The world, all-tackle angling record from Blackstone Harbour, Georgian Bay, Ontario was caught on 16 October 1988 by Kenneth J. O'Brien and weighed 29.48 kg and was 1.47 m long. A fish 62 inches (157.5 cm) caught in the Ottawa River 12 July 1997 was a world record in the live release category.
The Ottawa River is designated as a "record class fishery" since it has the potential to produce exceptionally large fish. The minimum size limit is set at 137.1 cm (54 inches).
Muskies are said to reach 55 inches (1.4 m) in the Rideau River (Wachelka et al., 2000) but see below. The Ottawa River may have fish weighing in the 62 lbs range, based on an estimation by a local angler of a fish he released ( https://secure.cccpdc.com/allcanada/html/canadianMemoriesInFisherman.cfm, downloaded 20 June 2003) and up to 62 inches in length (Kerr, 2004; not in the angler diary below). Kerr (2010b) summarises sizes of Muskies caught in the Ottawa River; the following was compiled before that work appeared.
Small (1883) reported the largest fish offered for sale by Mr. Lapointe in the NCR was 42 lbs (19.1 kg). Bebee (2004) records Muskies of 53 lbs 4 oz from Beckett's Landing on the Rideau River (1947) and shows photographs of 52 and 54 inch Muskies from the Long Reach of the Rideau River. Anonymous (1964) reported a 50 inch 35 lbs Muskie from the Rideau River near the Jock River. Waddell (1999) reported a 53 inch (1.35 m) Muskie with a 22½ inch (57.2 cm) girth caught trolling in the Ottawa River. Its estimated weight was 33 lbs (15 kg). Mullington (1999) reported a 58 inch (1.47 m) Muskie from the Ottawa River near Orléans, presumably the same fish as the 58 inch and 28.5 inch girth fish caught by Ed Barbosa on 20 October 1994 (www.canadian-sportfishing.com/NationalFishRegistry/Live_Release1.asp, downloaded 13 June 2003). A 53 inch Muskie was reported from Wendover in the Ottawa River (www.fish-hawk.net, downloaded 20 May 2003). Anonymous (1979) reported a 30 lbs 6 oz (13.8 kg) Muskie which was 47½ inches (1.21 m) long from the Rideau River. A 36 lb fish was caught in Dow's Lake in the 1960s (www.ottawafishing.com, downloaded 20 May 2003). Wachelka (1999) however gives details of mis-reporting a 55 lbs (25 kg) fish from Rockland on the Ottawa River (probably a 35 lbs fish (15.9 kg) with rumours of capture on the Rideau River. Newspaper reports have a 51.25 lb Muskie from near Rockland in the Ottawa River, caught in November 1996. A 62.25 x 27.5 inch Muskie weighing approximately 57 lbs was caught near Hull in the Ottawa River on 12 July 1997 (www.muskiescanada.ca, downloaded 20 May 2003). A 44.5 and a 55 inch Muskie from the Ottawa River is pictured in Kerr (2004). A 51 x 22 inch (1.3 x 0.6 m) Muskie was caught at Rockland 17 October 1998 by Rick Collin as were two 50 inch (1.27 m) fish by other anglers (The Release Journal, 22(1):5, 1999). A 51 inch Muskie from near Rockland was pictured at www.fishontario.com/photo_gallery/page1/Dan-Maclean.jpg, downloaded 23 August 2004. A 52 inch, 22 inch girth and 51.5 lbs weight Muskie was caught at Rockland by Brent Luckman (Mullington, 1998; Buie, 1998) and fish to 30 lbs are caught in bays near Clarence on the Ottawa River. A 56.5 inch Muskie with a girth of 26.5 inches and a reported weight of just over 51 lbs or 22.95 kg (or 23.15 kg converting the 51 pounds to kilogrammes) was caught near Kars in the Rideau River on 8 October 2004 (Ward, 2004). A 40 lb Muskie is reported as having been caught from the bay below Jessups Falls on the South Nation River (Naomi Langlois-Anderson, South Nation Conservancy, pers. comm., 7 August 2007). A 51-inch fish was found during a a census of winter-killed fish in Dow's Lake (Laucius, 2009).
Beebee (2007) lists old records of Muskie taken in the Rideau River, up to 58 lbs (26.3 kg) and 60 inches (1.52 m).
Weight in pounds for large Muskie released alive can be derived from the formula "girth2 x length/800", or allowing for overestimates for very large fish, the same formula can be used but ¾ inch should be taken off the girth before it is squared (The Release Journal, 22(1):16, 1999).
Found from southwestern Québec and southern Ontario west to western Lake Superior but only south of this lake to western Ontario and introduced to southern Manitoba. In the U.S.A. south to northern Georgia. The Chat's Lake area near Arnprior had 10,000 young Muskie stocked in 1956 (newspaper reports). The Rideau River was heavily stocked with this species in the 1940s and early 1950s (Hopkins, 2000). 25,000 fish were released in the Rideau River in the Long Reach near Kars or Osgoode in 1941 (newspaper reports vary; Bebee, 2004). The Jock River and Steven Creek (North Gower) have also been stocked with this species (Kerr, 2001a).
Origin
The Muskellunge entered the NCR from a Mississippian refugium.
Habitat
Muskellunge are found in the warm shallows of lakes and rivers where there is abundant vegetation such as pondweed and of cover such as fallen trees and boulders. Their requirements are narrower than those for Northern Pike. Detailed habitat requirements are given by Kerr (2010b).
The Jock River is the only natural small stream harbouring this species in southeastern Ontario (Billington, 2002) although the major rivers in the NCR are famous for their Muskie. Radiotelemetry indicates that there are about 50-60 Muskies between Hartwell Locks and downtown Ottawa in the Rideau Canal and the occasional large fish can be seen sunning itself from the canal path (Laucius, 2009). In the Carillon stretch of the Ottawa River near the eastern edge of the NCR, radiotelemetry showed 12 of 40 fish remained in lentic habitats (bays), 17 used mainly lotic habitats (main channel of the Ottawa River and tributaries) and 11 fish used both habitats (Hydro-Québec, 1996). Muskellunge used habitats deeper and further from shore in summer but closer to macrophyte stands than in spring. Greater distances were travelled in spring and autumn than in summer and winter. Young-of-the-year Muskies in the Carillon stretch occupied 3-5 m wide macrophyte beds in the upper part of the water column in contrast to pike which used the deeper portion. Relatively good water clarity is required for detecting prey (>1-2 m). Large fish often occur in deeper water along rocky shores, down to 12 m. They are more common in slower streams and rivers than Northern Pike. They are tolerant of high temperatures up to 32°C and low oxygen but prefer >4 mgL-1. However they prefer temperatures below 26°C, with 20-25°C optimal for growth and 6-12°C for spawning. They are said to prefer 25-28°C in summer. pH 5.0-9.0 is tolerated. A fish kill along extensive stretches of the Rideau River and Canal was reported in spring 1956 (newspaper reports).
Age and Growth
Life span has been estimated at over 30 years but even trophy fish are mostly less than 23 years old. A small sample from the Ottawa River had a maximum age of 20 years for 5 females and 17 years for 3 males (Casselman et al., 1999). Ottawa River fish are known for their longevity and for attaining trophy size. Growth is rapid in the first few years but the rate varies with locality being slower in northern Ontario than the south. Both sexes mature at 3-6 years although males are smaller. Females generally live longer than males.
Food
Small Muskellunge up to about 4 cm feed on zooplankton but soon switch to fishes alone. Young Muskies are eaten by large insects and other fishes. In The Ottawa River Muskies are known to feed on Mooneye when they enter shallow waters. Fishes are seized by a rapid dart from motionless concealment in vegetation or near fallen logs and stumps. A Muskie may watch a prey item for many minutes before striking. The prey is impaled crossways on the large canine teeth, taken back to concealment and rotated in the mouth so it can be swallowed head first. This prevents injury if the prey has spiny fins. As well as fish, frogs, crayfish, snakes, muskrats, mice, shrews, chipmunks and various waterfowl are taken. Young and adults are cannibals; a 71.1 cm fish was found to have a 40.6 cm Muskie in its stomach. They are reported to make occasional lunges at bathers who claim to have been bitten and there is a report from the 1956 Ottawa Citizen of a Muskie grabbing the leg of Melville McConeghy while he was dangling it in the water from a boom of the Gillies Lumber Company near Arnprior in the Ottawa River. Reported attacks are on dangling hands or legs and swimmers, being too large, are not attacked.
Reproduction
Spawning occurs in April to May after ice melt and later than Northern Pike. Peak spawning in the Carillon stretch of the Ottawa River near the eastern edge of the NCR peaked at 27 April-5 May (Hydro-Québec, 1996). Kerr (2010b) records several spawning areas for Muskies in the NCR reach of the Ottawa River although this has not been well investigated. Water temperature is usually over 9°C in shallow, vegetated or debris-rich flooded areas. The Kemptville Creek estuary wetland is a regionally significant spawning site (Schueler et al., 1996). The fish home to specific spawning grounds and to areas on these grounds. Some fish have been caught at the same place for 7 years in Stony Lake, Ontario. Such fish may well have summer home ranges distinct from other spawning groups and are reproductively isolated. This could be very important in managing Muskellunge stocks. The large female swims over the vegetation with one, sometimes two, males, both sexes rolling to bring their genital areas close together when eggs and sperm are shed as the fish vibrate. Their tails lash to spread the adhesive eggs which fall into the vegetation. The spawning season usually lasts a week with many such spawning acts. A female may have 265,000 amber eggs up to 3.5 mm in diameter. Muskies hatch in 13-15 days at 13°C.
Importance
Commercial landings of Muskellunge in Ontario and Québec reached an estimated 447,578 fish in 1888-1897 but commercial fisheries were closed in 1936 to protect the developing sport fishery. Dymond (1939) records catches from 1875 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 26,650 lbs (12,099 kg) in the Ottawa River from Carillon to Pontiac in Québec) including the Gatineau lakes, the highest recorded. On the Ontario side the highest catch was in 1891 at 12,050 lbs (5471 kg) from the Ottawa River fronting on Prescott, Russell and Carleton counties and inland waters.
This large sport fish is avidly sought by residents and visitors to Canada and it is a mainstay of the angling industry. The Ontario Muskie sport fishery appears to be stable and sustainable (Kerr, 2007)). Large Muskellunge are more common in Canadian than U.S. waters, including waters of the NCR. The Ottawa Chapter of Muskies Canada has carried out radio-tracking in 1994-1997 and is involved in habitat restoration to protect this sport fish (Smith in Kerr, 1999b). A Muskies Canada sponsored contest on the Ottawa River in August 1998 resulted in 33 fish being caught by 35 anglers, the biggest fish being 51 inches (1.3 m) long (Smith in Kerr, 1999b). The lower Ottawa River below Rockland attracts anglers from the United States for the large Muskie found there and the Rideau River has the best catch rate in eastern Ontario, remarkable for a large metropolitan area (Hopkins, 2000).
An estimated 100 person-hours of angling is required to capture a Muskie large enough to keep. However 74 keen Muskie anglers in Lake St. Clair caught 1273 fish in 1017 days of fishing. There are a number of fishing clubs in Canada and the U.S. devoted to catching Muskellunge. They are caught by trolling or by using a large, live fish as bait. They are very strong fighters and will leap out of the water. There are strict fishing regulations in regard to size, season and bag limits in order to maintain stocks of this species (OMNR, 2002; Casselman, 2007). In Ontario in 1999, competitive fishing events encouraged live release after measuring length in the water at the point of capture and an honour system for reporting catches (Smith in Kerr, 1999b; Kerr, 1999d). However about 30% of all angled Muskellunge die because of stress, including many apparently fit fish released by anglers. The flesh is white and flaky and Muskie can be baked, poached or fried but most larger fish are mounted as trophies. Environnement Québec has a recommended limit of 4 meals per month from large-sized Muskie taken in the Ottawa River below Gatineau (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Angler diaries provide the following data summarised in Kerr (2004). Dow's Lake and the Jock River are within the NCR but the
Ottawa and Rideau River reports presumably include fish from outside the NCR. Note that not all years are reported in the range given
and completeness of reports will vary within years:-
| Water Body/Years | Angling effort (rod hours) |
Catch (Number of fish) |
Harvest (Number of fish) |
CUE* | Mean size# of angled fish (and sample size) |
Largest fish# |
| Dow's Lake/1995-2003 | 379.25 | 67 | 0 | 0.177 | 34.9 (67) | 45.0 |
| Jock River/1988-2002 | 262.50 | 57 | 0 | 0.217 | 30.2 (44) | 39.0 |
| Ottawa River/1981-2003 | 16,074.75 | 1549 | 0 | 0.096 | 37.6 (1390) | 58.3 |
| Rideau River/1985-2003 | 3442.60 | 434 | 0 | 0.126 | 34.5 (400) | 55.0 |
* = catch per unit effort
# = in inches
These figures show that many serious anglers release their catch unharmed, your chances of catching a Muskie are better in the Jock but the big ones are in the Ottawa with large fish in the much smaller and more accessible Rideau.
Umbridae - Mudminnows - Umbres
Mudminnows are freshwater fishes of the Northern Hemisphere found in Alaska, central and eastern North America, and in Europe and Siberia. There are only 7 species with 1 reported from Canada and the NCR.
They are characterised by the dorsal and anal fins being far back on the body near the tail as in the Pike Family, no adipose fin, a toothless maxilla bone in the upper jaw, no pyloric caeca (finger-like gut branches), branchiostegal rays 5-8, rounded tail fin, absence of a duck-billed snout and no groove between the upper lip and the snout.
The closest relatives of this small, innocuous fish are members of the large, predatory Pike Family.
Mudminnows, as their name indicates, are found in muddy or swampy areas. They can survive low oxygen conditions by breathing air. They can survive drying by burying themselves in the soft ooze and detritus. It is doubtful that they can survive complete desiccation of the habitat. There is evidence that some species can survive being frozen in ice and certainly the Canadian species remain active in winter under an ice cover, using oxygen directly from gas bubbles under the ice.
Central Mudminnow / Umbre de vase
Umbra limi (Kirtland, 1840)
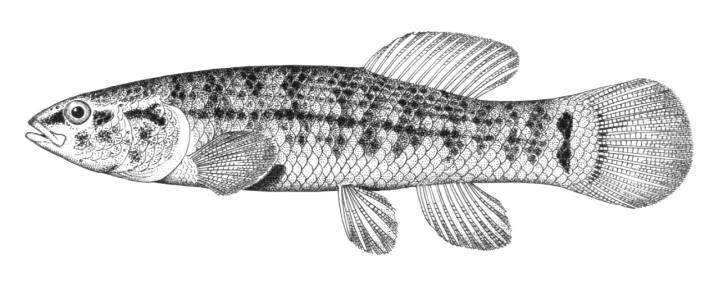
Taxonomy
Other common names include Western Minnow, Mudfish, Mudpuppy, Dogfish and Mississippi Mudminnow.
Key Characters
This is the only species in the family in Canada and is easily recognised by the anal and dorsal fins being close to the tail, absence of a duck-like snout and by the vertical bar at the tail fin base.
Description
Dorsal fin rays 13-17, anal rays 7-10, pectoral rays 11-16 and pelvic rays 6-7. There is no lateral line and lateral scales number 30-39.
Colour
The head and body are brown, black or olive-green above, the flanks brown with traces of numerous bars in some fish and the belly white to yellowish. Fins are brown, sometimes tinged with pink or orange. Spawning males have iridescent green or blue-green anal and pelvic fins. The peritoneum is light brown with darker speckles.
Size
Size attained is 13.2 cm standard length (and 14.8 cm total length).
Found in central North America west of the Appalachian Mountains south to Tennessee and Arkansas. In Canada it is found in southern Manitoba and adjacent areas of western Ontario and south of a line from the southeastern corner of Lake Superior across to about Québec City.
Origin
This species is entered the NCR from a Mississippian refugium.
Habitat
This species inhabits weedy and detritus-rich streams, ditches, bogs and small ponds where oxygen content may be low compares to other fish habitats. They may also be found in lakes. The bottom is usually mud and detritus but can be gravel and stones. Small (1883) reports them hovering in mid-water using the pectoral fins and darting violently to the surface and back at long intervals, presumably to gulp air.
Age and Growth
Females live longer than males and may attain 9 years of age, but this is uncertain as Mudminnow scales are difficult to "read" for age. Maximum age may be only 4 years. Males are mature at 1-2 years of age and females at 1-3 years.
Food
Mudminnows feed on bottom-dwelling animals such as crustaceans, insect larvae, worms, molluscs, rarely small fish but certainly other Mudminnows, and other small inhabitants of weedy, detritus-rich ponds and small creeks. Curiously only female Mudminnows ate fish in a Manitoba study and older females consumed mostly fishes. During winter this diet may contribute to egg development so that spawning can occur soon after ice break-up, and so young have longer to grow before facing their first winter. Prey is attacked by a swift dart from concealment in vegetation. Mudminnows are common prey for many larger fishes such as catfishes and members of the Pike and Sunfish families. The Northern Water Snake (Nerodia sipedon) eats this fish in Ramsay Lake, Gatineau Park (McMurray, 1984). Even birds, muskrats and foxes have been recorded as eating them.
Reproduction
This species moves upstream, onto shores or into ponds and marshes to spawn. Thellen (1994) gives a spawning temperature of 13°C for the Outaouais. Spawning occurs in early spring over flooded areas and up to 2286 eggs are produced which stick to vegetation and are abandoned by the parents. A female from the NCR caught on 19 April contained eggs 1.4 mm in diameter.
Importance
They have been used as bait fish and are excellent aquarium fishes as they do not require an air pump.
Smelts are found in coastal sea waters and freshwaters near the coast of the Northern Hemisphere. Some populations are land-locked. There are about 11 species with 9 reported from Canada and 2 from the NCR.
These small, silvery fishes have a single dorsal fin at mid-body, an adipose fin, no pelvic axillary process (found in related Salmon Family members), 8 pelvic fin rays and 19 principal caudal fin rays, forked tail, 6-10 branchiostegal rays, 0-11 pyloric caeca, a stomach sometimes with a blind sac, the last vertebra at the tail is turned up, teeth are on numerous mouth bones and are strong or weak, a mesocoracoid bone is present in the pectoral fin skeleton and the skull lacks the orbitosphenoid bone. Scales are cycloid and easily detached. Some smelts have a strong cucumber smell when fresh, the chemical being trans-2-cis-6-nonadienal. Its function is unknown.
Breeding smelts can develop tubercles, modified scales and enlarged fins. Reproduction may occur in coastal waters or involve a migration into freshwater. Smelts are all carnivorous fishes. They are very numerous and so are important food fishes for commercially important species and are also used as bait by anglers. They are rich in oil and are excellent smoked for human consumption.
Fossils of Mallotus villosus (Müller 1776), another smelt species, are very common in nodules from Champlain Sea deposits throughout the NCR (Arsenault, 1979; McAllister et al., 1987).
Rainbow Smelt / Éperlan arc-en-ciel
Osmerus mordax (Mitchill, 1814)
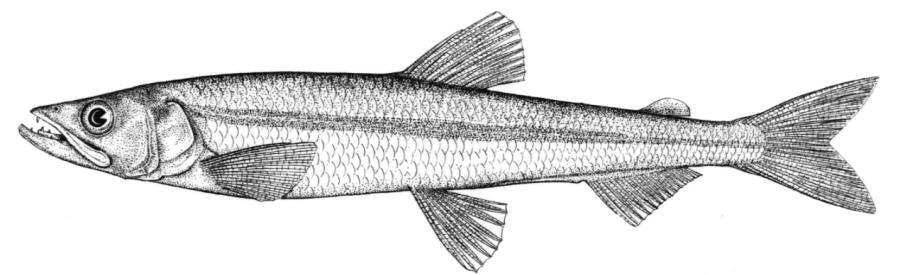
Taxonomy
Other common names include American Smelt, Atlantic Smelt, Atlantic Rainbow Smelt, Arctic Rainbow Smelt, Freshwater Smelt, Saltwater Smelt, Frostfish, Icefish, Leefish, Lake Smelt, Spirling, Sea Smelt, Éperlan du nord and Éperlan d'Amérique.
Key Characters
This species is distinguished by the presence of an adipose fin but it lacks the pelvic axillary scale found in the Salmon Family which it resembles. It is separated from the only other smelt in the NCR by usually having up to at least 200 mm standard length, having 8-14 upper arch and 15-24 lower arch gill rakers (usually 28-32 total), and orbit diameter 7.0-11.3% of standard length.
Description
Dorsal fin rays 8-11, anal rays 11-17 and pectoral rays 9-14. Lateral line scales 56-72 with 13-30 pored. Total gill rakers 26-37. There are medium conical to large canine teeth on the tongue, 4-9 pyloric caeca, and 1-3 large canines on each side of the vomer bone in the roof of the mouth. Males have numerous head, body and fin tubercles. The mouth is large and reaches the rear of the eye. Paired and anal fins are larger in males and a lateral ridge may develop. The right ovary and right testis in this species are significantly smaller than the left (Legault and Delisle, 1968).
Colour
The back is olive-green, steel blue or black and the flank is silvery with purple, blue or pinkish iridescences (hence "rainbow"). Scales on the back are outlined with pigment. There is a silvery stripe along the flank which turns dark in preserved fish. The peritoneum is silvery with dark pigment dots. Landlocked smelt are darker than other populations and may have dusky fins rather than the typical clear fins. Faber (1985a) illustrates a larva.
Size
Reaches 35.6 cm.
Found from southern Labrador throughout the Maritimes south to New Jersey in the sea. Also enters rivers and is landlocked in lakes throughout eastern Canada. Introduced and widespread in all the Great Lakes and adjacent inland lakes including Lake Winnipeg (also introduced in the Mississippi River basin). In the Arctic from Cape Bathurst, N.W.T. coastwise around Alaska and south to Vancouver Island but relatively rare in Pacific Canada.
Origin
Anadromous smelt require summer sea temperatures above 10°C and this would prevent invasion of the Champlain Sea until about 11,000 B.P At this time sea level in the NCR had fallen to a present-day level of 450 ft and so smelt could only enter lakes in the central Gatineau Valley. Lakes in the southern Gatineau Valley above 450 ft were inaccessible (Dadswell, 1974).
Fossils of Quaternary age (ca. 10,000 years ago) have been found in clay nodules from Green Creek in the NCR (McAllister et al., 1981; Harington, 1972; 1983; McAllister et al., 1987).
Habitat
Rainbow smelt are found as landlocked populations in numerous lakes of various sizes, although deep and thermally stratified in summer. In lakes cool water is preferred, about 7°C, although some reports give 15°C as the preferred temperature for the species. They are not attracted to lights at night nor does they make vertical migrations in Heney Lake in contrast to Pygmy Smelt (Delisle, 1969a). They are pelagic with a maximum recorded depth of at least 150 m in the sea. In the Great Lakes they are commonest at 18-60 m and rarely descend below 75 m. They also occur in rivers. This smelt is susceptible to acute infections with Glugea hertwigi, a sporozoan parasite, in the NCR. White cysts of the parasite cause the intestines to adhere together and the fish starve (Delisle, 1965a; 1965b; 1969; Legault and Delisle, 1967; Delisle et Veilleux, 1969).
Age and Growth
Females live longer, grow faster and are larger than males. Life span is 5-9 years in most populations but up to 17 years on the Beaufort Sea coast of the Yukon. Maturity is attained at age 3 in Atlantic populations although some fish mature a year earlier. Alaskan and northern Canadian populations mostly mature at ages 4-7, often at 6-7 years and 20-22 cm. In Heney Lake most fish reach maturity at 3 years with a maximum life span of 6+ years (Delisle, 1969a).
Food
Food is often opossum shrimps and other invertebrates such as worms and insects in fresh waters. Over 50% of the diet in Heney Lake is adult Pygmy and young Pygmy and Rainbow smelts (Delisle, 1969a). Many fishes in lakes depend on smelt as a food source including such economically important species as Atlantic Salmon, Brook Trout, Lake Trout, Walleye and Yellow Perch.
Reproduction
Spawning migrations take place February to June, being later in northern seas and lakes. The run usually starts when water temperatures reach 4°C or higher, just after the ice breaks up and moves out. Lake populations enter rivers even under ice. Spawning itself takes place at 5-9°C in various localities in Ontario. Spawning in Meech Lake, Québec was observed on 19 May (Bridges and Delisle, 1974) but these may be Pygmy Smelt. Deepwater spawning takes place in Lake Heney, north of the NCR. Temperatures here are below 4°C in March-April (Legault and Delisle, 1968; Delisle, 1969a; Lanteigne and McAllister, 1983). Thellen (1994) gives a range of 8.9-18.3°C for fish in the Outaouais generally. Males are first on the spawning grounds in freshwater areas of rivers. Spawning occurs at night. Eggs are adhesive, 1.0 mm in diameter, and number up to 93,000 per female. The outer membrane of the egg ruptures but remains attached to the egg by a stalk. This outer membrane is the adhesive part and the egg sways in the water at the end of the stalk. They become attached to vegetation or rocks and may be so numerous that some are smothered. As many as 190 eggs/sq cm have been counted. Larvae are 4.0-5.0 mm at hatching. The fry are carried downstream to the lake after hatching.
Importance
Mass mortalities of adults have been reported in the Great Lakes after spawning, 200 m long, 4.6 m wide and 0.3 m deep. This creates a health hazard, renders beaches unusable and is costly to clean up. Smelt are important in both sport and commercial fisheries. They can be legally seined or dip-netted at night on the spawning run, caught on hook and line from docks, or through the ice in winter. Commercial catches are taken in the Great Lakes using otter trawls and in the Miramichi River estuary by box nets through the ice. Bag nets, fyke nets, gill nets and seines are also used. In 1979 2542 tonnes worth $1,065,767 were caught in Atlantic coastal areas of Canada and 10,979 tonnes in the Great Lakes worth $2,035,000. Ontario had a catch of 7289 tonnes in 1966 and smelt occupied first place by weight among all Great Lakes fishes in that year. The total Canadian catch in 1988 was 11,000 tonnes worth about $4.2 million. Smelt are sold fresh, frozen or pre-cooked, are delicious fried and are in high demand by consumers in Japan and the U.S.A. Their cucumber-like odour when fresh is distinctive and probably accounts for the name "smelt". Fishermen have claimed that smelt are important predators on commercially important species. Although fish are a relatively small part of the smelt diet (as little as 4.3% for males in Lake Simcoe), the numbers of smelt alone lend some weight to this accusation. Whether the numbers of young commercial fishes eaten by smelt are offset by the numbers of smelt eaten by survivors remains to be determined. Rainbow smelt have been widely distributed in southern Ontario as bait fish introductions.
Pygmy Smelt / Éperlan nain
Osmerus spectrum Cope, 1870
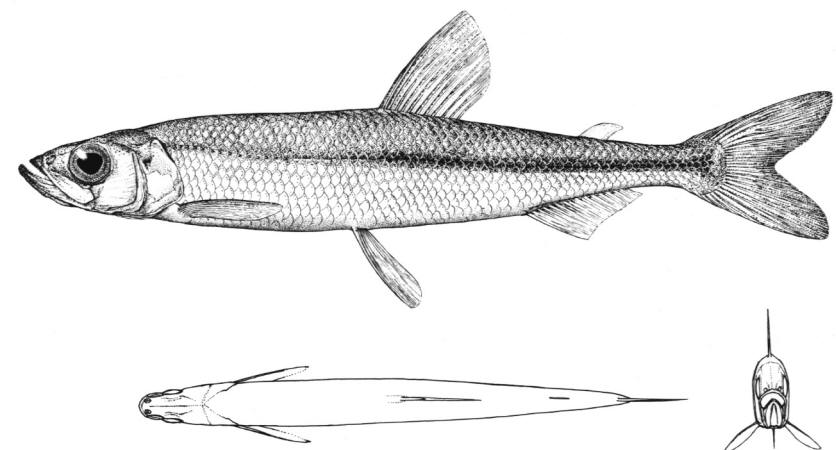
Taxonomy
Recent studies maintain that the dwarf and large forms of smelt in lakes should be regarded as stocks rather than species (Lajoie, 1986; Bernatchez and Giroux, 2000). This form is retained here with a separate account for its local interest.
Key Characters
This smelt is distinguished from the other lake smelt in eastern North America, the Rainbow Smelt, by being less then 13.5 cm standard length, usually 34-36 (range 32-36) gill rakers and eye diameter 4.4-6.5% of standard length (Delisle et Veilleux, 1969; Copeman, 1973; 1977; Lanteigne and McAllister, 1983).
Description
In addition to the key characters above, mid-lateral scales number 58-66 and vertebrae 59-63.
Colour
Overall coloration silvery. Preserved fish have a dark flank stripe and speckled fins with the anal fin being almost clear.
Size
Attains 22.9cm (Cuerrier, 1983).
Found in Heney Lake, Québec, Lake Utopia, N.B. and in lakes of southern Maine. Probably in various other Québec, N.B. and Maine lakes. Introduced to Meech (or Meach) and Ouimet lakes, Québec from Lake Utopia (Lanteigne and McAllister, 1983). Dymond (1939) records that the Meach Lake fish were introduced as half a million eggs in each of two successive years about 1924, a few specimens were subsequently reported but the species was then believed to be extinct. Records from the Ottawa River and Québec lakes (other than Meech) mapped here may well be mis-identifications.
Origin
Presumably from an Atlantic coastal refugium for those local stocks that are native.
Habitat
This smelt lives in lakes along with the larger Rainbow Smelt. The lakes are small, deep and thermally stratified in summer. Delisle (1969a) and Lanteigne and McAllister (1983) summarise biology in Heney Lake (= Lac Poisson Blanc) north of the NCR. The population size of 2-3 years olds there has been estimated at 22 million fish by Delisle but it may not have been as common by 1984 (McAllister et al., 1985). Adults migrate in July from the hypoliminion at the bottom (at 7-9°C) to the epiliminion (at 21°C) at 2300 to 0200 hours. Adults form schools beneath the ice in January. Young pygmy smelt are found in schools and are attracted to artificial lights at night. Young-of-the-year have been reported in shallow water in July, August and winter near sand and gravel beaches. They migrate from deep water to the surface between 0100 and 0400 hours in April before spawning. They are susceptible to the same sporozoan parasite as Rainbow Smelt with a massive spring-time mortality of at least 2-3 million fish (McAllister et al., 1985).
Age and Growth
Maturity is reached at age 2 and life span is 5+ years although only 2.2% reached this maximum in Heney Lake north of the NCR. Most spawners are 2 years old. Growth is slower in Pygmy Smelt than in Rainbow Smelt (Delisle, 1969a; Copeman, 1977).
Food
Food is mainly plankton such as adults and eggs of water fleas (Daphnia) and adult Cyclops and Bosmina in Lake Heney north of the NCR (Delisle, 1969a) but may also include insects and amphipods.
Reproduction
Spawning occurs in April and May during and after spring breakup, and later than Rainbow Smelt in Lake Heney, north of the NCR and in transplanted fish in Meech Lake in the NCR. Temperatures are 4-8°C (average 6°C) and eggs are shed on sand and gravel beaches (Delisle, 1969a; Bridges and Delisle, 1974). Up to 3774 eggs of 0.7 mm diameter are found in preserved females (Delisle, 1969a).
Importance
The Committee on the Status of Endangered Wildlife in Canada is considering a status of "rare" for this species. Numbers of Pygmy Smelt in Heney Lake appear to have declined. Millions of eyed eggs of this species taken from Meach Lake in Gatineau Park in May 1965 and May 1966 were introduced to Papin Lake in Pontiac County (76°06'N, 46°09'W) just outside the NCR as food for Ouananiche (introduced landlocked Atlantic Salmon)(Cuerrier, 1983).
Salmonidae - Trouts and Salmons - Truites et Saumons
The salmons, trouts, charrs, graylings, whitefishes, ciscoes and their relatives are found in the cool and cold, fresh and salt waters of the Northern Hemisphere. There are about 70 species with 37 reported from Canada, and 9 in the NCR. Salmons are medium to large sized fishes up to about 1.5 m.
These fishes are characterised by having an adipose fin, 11-222 pyloric caeca, the swimbladder is connected to the gut by a duct, the gill membranes are free of the isthmus and extend far forward, the last 3 vertebrae are turned up in the tail skeleton, the eye muscles pass through a deep posterior myodome and attach to trunk muscles and there are 7-20 branchiostegal rays. Scales are cycloid, confined to the body and can be minute (and hard to see at a superficial examination) or moderately large (and fairly obvious at a glance). Pelvic, and sometimes pectoral, axillary scales are present. Pelvic fins are abdominal in position. There are no fin spines and the dorsal fin is at mid-body. The lateral line is obvious. Breeding tubercles develop in the spawning season in some species. Many have strong teeth on both jaws, the tongue and the roof of the mouth. These fishes are tetraploids.
The limits and relationships of species within the family have long been in contention. A number of species exist which have not been named while others have a name but contain more than a single species. The latter are often referred to as a species "complex" since it is difficult to describe the complex observed variation in simple terms of several, named species. Even the correct scientific name to apply to a species can be in dispute or not open to easy resolution. The familiar Rainbow Trout, long known by the scientific name of Salmo gairdneri, is now generally recognised as Oncorhynchus mykiss, a relative of the Pacific salmons rather than salmon and trouts of Europe. The word "salmon" is from the Latin for Atlantic Salmon. Young salmons are difficult to identify as their appearance changes with growth and adults can show colour and other character variations related to locality and season.
The salmon family is extremely important for the sport and commercial fisheries it supports. The commercial salmon landings in British Columbia for 1988 weighed 88,000 tonnes worth $312 million. When combined with the fish processing industry the salmon were worth probably over $1 billion and in addition there is an important sport fishery and farming operations. Many thousands of books and scientific papers have been written about these fishes. Members of the family have been widely introduced elsewhere in the world in cool waters, including the Southern Hemisphere. Certain salmonids are entirely resident in fresh water but many are anadromous, spending their life at sea but running up rivers to spawn and die. Migrations can be over thousands of kilometres and adults return to their stream of birth, a remarkable example of accurate navigation and ability to cross obstacles like falls by jumping. The young develop for several years in fresh water. Young salmons, trouts and charrs are known as fry or alevins, when a few centimetres long they have dark blotches (parr marks) along the flank and are called parr, as they run to the sea they become silvery and are called smolt, an adult male returning early from the sea to freshwater is a grilse, and in the Atlantic Salmon, which can return to spawn several times, the spent fish returning to the sea are called kelt.
A decline in the abundance of Lake Trout, Brook Trout, Cisco and Lake Whitefish in Gatineau Park over the previous hundred years was noted by Rubec (1972; 1975). The causes are overexploitation by commercial fishermen, poaching and angling. Low oxygen levels in the hypolimnia of most Gatineau Park lakes is also major factor limiting salmonids, perhaps resulting from deforestation allowing more nutrients into the lakes coupled with increased human presence and associated wastes.
Bergeron and Brousseau (1982) and Bernatchez and Giroux (2000) map Prosopium cylindraceum (Pennant, 1784) within the NCR but this is not confirmed by specimens.
Cisco / Cisco de lac
Coregonus artedi Lesueur, 1818
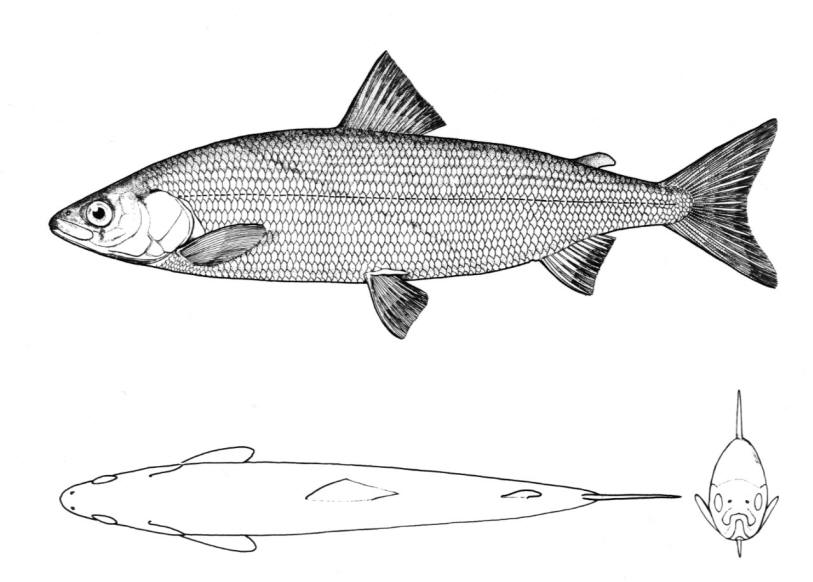
Taxonomy
Other common names include Lake Cisco, Lake Herring, Tullibee, Freshwater Herring, Blueback, Sand Herring, Shallowwater Cisco, Grayback Tullibee, Bear Lake Herring, Blueback Tullibee, Herring Salmon, Hareng de lac, Arnaqsleq, Kapisilik and Kaviselik. This species shows considerable variation over its wide range and some variants have been given scientific names, 24 subspecies being described in 1931. The confusion of genetic identity through man-induced changes and extinction of some forms and lack of study makes a resolution of these names difficult. In some lakes there are two forms, a small and a large one with different biology and anatomy. Cisco have been referred to as a Coregonus artedi "complex" which indicates that there is not a single, clearly defined species but a complex of forms. There is also a spring spawning population in Lac des Écorces, to the north of the NCR in the Lièvre River basin which, being reproductively isolated from the normal fall spawning population, can be regarded as a distinct species (see references by M. Hénault and R. Fortin, and Pariseau et al. (1983)). Hybrids with Lake Whitefish are reported for Canada, further confusing some identifications.
Key Characters
This member of the Salmon Family is distinguished from its relatives by having large, silvery scales (94 or less), no parr marks in young, teeth absent from the jaws and roof of the mouth, forked tail fin, 2 flaps of skin between the nostrils, the upper and lower jaws are about equal, giving the front of the head a pointed appearance (and the mouth is not under the snout), and gill rakers 38-64, usually 46-50.
Description
The lower jaw is usually about the same length as the upper jaw which extends back to the anterior eye margin, there is a knob at the lower jaw tip, the premaxilla bone of the upper jaw curves backwards, the body is deepest at the middle and is elongate and rounded in cross section. Dorsal fin rays 9-15, anal rays 10-15, pectoral rays 14-18 and pelvic rays 8-12. Lateral line scales 63-94. Pyloric caeca number about 100. Males have one nuptial tubercle in the centre or near the edge of each scale.
Colour
Overall colour is silvery with pink to purple iridescence. The back may be grey, light brown, bluish, greenish or almost black and the belly is white. The dorsal and caudal fins may be darkly pigmented on the distal half or on the margin. Pelvic and anal fins are milky. The pelvic fins have dark tips. Fins are generally clear with limited pigmentation.
Size
Reaches 57.2 cm total length and 3.63 kg. The Ontario record as of the year 2000 weighed 2.0 kg.
Found from eastern Québec, including Ungava Bay and southern Hudson Bay west to northern Alberta and much of the N.W.T., north to Great Slave Lake and the lower Mackenzie River. Also in U.S. states adjacent to the Great Lakes. Specimens were collected from Lac LaPêche in 2003 and are deposited in the Canadian Museum of Nature. A limited number of cisco were introduced to Meach Lake in the late 1960s (Rubec, 1972). Rubec (1972) has it in lakes Lapêche, Philippe, Mousseau and Meach in the late 1800s, absent today, although occasional fish are caught or found dead in Lac Philippe (e.g. in 2003 by A. Martel).
Origin
This species probably entered the NCR from a Mississippian refugium (Turgeon and Bernatchez, 2001) or possibly from a Mississippian or Atlantic refugium (Mandrak and Crossman, 1992).
Habitat
This is a widespread freshwater, lake species also known from some large rivers. Cisco are pelagic in lake midwaters, at 18-53 m in Lake Superior for example, with movements into deeper, cooler water in summer and into shallower waters in fall. There are reports of daily vertical migrations with cisco coming to the surface at night under winter ice to feed on crustaceans. It is rarely found at temperatures above 18°C and its preferred temperature is 13-18°C. Cisco are tolerant of some turbidity.
Age and Growth
Life span is 31 years of age and maturity is attained at 1-6 years depending on locality, later in the north.
Food
Food is mainly plankton and large crustaceans such as opossum shrimps and amphipods but can include aquatic insects and molluscs in shallow waters or terrestrial insects taken at the surface. Eggs of their own and other species are eaten, as well as fry and minnows. A variety of other fishes eat Cisco including Lake Trout, Northern Pike, Yellow Perch, Walleye and Burbot. Lake Trout depend on this species for growth as cisco are found in the deep water of the summer habitat of trout; without cisco, trout feed on small crustaceans and do not reach a large size. Strongly swimming prey is taken by a dart and suck action. Gulping is also used where the fish open and close the mouth 2-3 times per second, taking more than 1 prey at a gulp. Even buried prey can be taken.
Reproduction
Spawning takes place from September to December, earliest in the north, when large schools form, usually at 6°C or less, optimally at 3-4°C. The spawning ground is often gravel shallows at 1-3 m but can occur pelagically at 9-42 m over deep water. Spawning may occur under a thin ice cover and is therefore difficult o observe. Males arrive on the spawning grounds first, 2-5 days before females. As many as 12 males will follow a single female but at spawning time the female is usually accompanied by 2 males. She descends to 15-20 cm above the bottom, leading the males whose heads are level with her anus. Eggs are shed, mostly at night, fall to the bottom and are abandoned. The eggs are slightly adhesive and become attached to the bottom. Ripe females produce up to 29,000, 2.1 mm diameter eggs. These eggs hatch the following spring at ice break-up.
Importance
This cisco was once very abundant and of great commercial importance with as much as 21.8 million kg being taken from Lake Erie in 1918. Localised fisheries still catch this species for food, for fur farms and for dogs in the north. It is sold fresh, frozen or smoked and is very good eating. The total Canadian catch in 1988 was 1400 tonnes. Anglers may catch it on live bait including minnows, artificial lures and flies, and by ice fishing and spearing. Ice fishing is popular in the Outaouais. This species is used as bait for Lake Trout.
Cisco populations are declining. In addition to overfishing and other factors outlined under ciscoes, this species is sensitive to enrichment of lakes since it thrives in infertile waters. Enriched or eutrophic waters have depleted oxygen levels in summer in the deep, cool waters favoured by cisco. The cisco is forced into surface waters where temperatures are too high and summerkills are common.
A fossil of Quaternary age (ca. 10,000 years ago), possibly this species, has been found in a clay nodule from Besserer's Springs (near Green Creek at Hiawatha Park in the NCR) (McAllister et al., 1981; Harington, 1983; McAllister et al., 1987).
Lake Whitefish / Grand corégone
Coregonus clupeaformis (Mitchill, 1818)
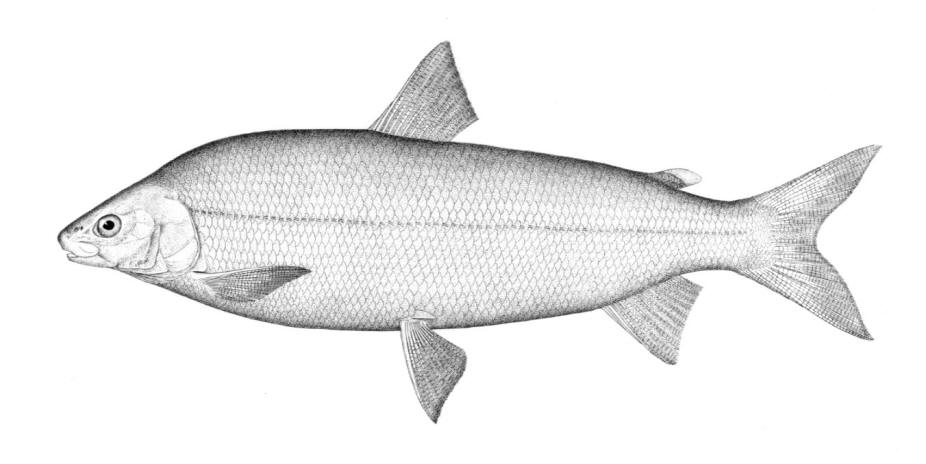
Taxonomy
Other common names include Common Whitefish, Sault Whitefish, Eastern Whitefish, Great Lakes Whitefish, Inland Whitefish, Gizzard Fish, Lake Herring, Labrador Whitefish, Sead, Humpback, Buffalo Back, Whitebait, Poisson blanc, Pointu, Pi-kok-tok, Jikuktok, Anahik, Kapihilik, Pikuktuuq, Kakiviatktok, Kavisilik, Anâdlerk, Kakiviartût, Keki-yuak-tuk, Anadleq and Qelaluqaq. This species has been referred to as the Coregonus clupeaformis "complex" because of its wide range in variation, particularly in northwestern North America, which may encompass more than one species. Some lakes, for example in Algonquin Park, Ontario, contain both dwarf and normal forms which differ in biology but not in anatomy, apart from size. In Lake Como, Ontario dwarfs are 170-179 mm and normals 280-289 mm fork length. In the Yukon several lakes have 2 forms of whitefish, a benthic form with low gill raker counts feeding on the bottom and a pelagic form with higher gill raker counts feeding on zooplankton throughout the water column. High raker fish have shorter life spans and mature earlier: they are the more unique member of the 2 forms. Samples from 5 Ontario lakes showed differences in gill raker numbers, pyloric caeca numbers, in size of the tail, dorsal fin and eye, and in biochemistry, evidence of genetic separation since they spawn at the same place and time. Evidence from mitochondrial DNA studies shows that different genetic structures correlate with the Pleistocene glaciations. Hybrids between Lake Whitefish and Cisco exist in the Great Lakes and elsewhere and are known as "mule whitefish". They are bright green in colour and intermediate in anatomical characters.
Key Characters
This member of the Salmon Family is distinguished from its relatives by having large, silvery scales (97 or less), no parr marks in young, teeth absent from the jaws and roof of the mouth, forked tail fin, 2 flaps of skin between the nostrils, the mouth is under the snout, and gill rakers 19-37.
Description
Dorsal fin rays 10-13, anal rays 10-14, pectoral rays 14-17 and pelvic rays 10-12. Lateral line scales 70-97. Pyloric caeca number 140-222. The premaxilla bone of the upper jaw curves backward making the snout rounded and the adjacent maxilla bone is twice or more longer than wide. Nuptial tubercles are found on 3 or more scale rows above the lateral line and 6 rows below, and sparsely on the head. Each scale has 1 central tubercle with smaller tubercles on each or either side on some scales. Large fish develop a hump on the back, particularly in northwestern Canada. Lake whitefish produce more mucus than Ciscoes and feel slipperier.
Colour
Overall colour is silvery with a light to dark brown, greenish or black back and a silvery-white belly. Scales are outlined with pigment in northwestern Canada. Flanks may have a bluish-tinge. Fins vary from clear in the south to black-tipped in the north but may be dusky. Lower fins generally clear but sometimes with dark spots. Faber (1985a) illustrates a larva.
Size
Reaches 91 cm total length (perhaps to 1.0 m) and 19.05 kg. The Ontario record as of the year 2000 weighed 6.7 kg.
Found from New Brunswick and some Nova Scotia lakes, and Labrador west to British Columbia and throughout the Yukon and N.W.T. including Victoria Island. Also introduced into Newfoundland and parts of British Columbia and Alberta. Also in Alaska and U.S. drainages of the Great Lakes and St. Lawrence River basin.It is rare in the NCR. Small (1883) reported it in many lakes of the Gatineau and Lièvre districts but these may have been north of the NCR limits. Rubec (1975) has it in lakes Meach and Mousseau in the late 1800s, surviving today only in Lake Mousseau. Lake Whitefish have been propagated in Ottawa area hatcheries as early as 1890 (Lasenby et al. 2001).
Origin
This species entered the NCR from a Mississippian refugium or possibly from an Atlantic, Beringian or Missourian refungium (Mandrak and Crossman, 1992).
Habitat
Lake Whitefish are found, appropriately enough, in lakes but are also in large rivers such as the Lac Deschênes section of the Ottawa River. Generally they are found close to the bottom but may be pelagic. They prefer large, cold lakes, depths >12 m for non-spawning periods and 6-14 m for spawning, temperatures of 11.9-17.0°C (preferred temperature is 12.7°C), and less than 6°C for spawning, substrates of gravel, stones and sand for spawning, and a pH greater than 5.4. They can tolerate high levels of total dissolved solids (>3000 mg L-1). Their depth range depends on temperature. In southern Canada they retreat to cooler, deeper waters in summer, down to at least 128 m but in northern Canada summer temperatures are probably cool enough in surface waters.
Age and Growth
Females live longer and mature later than males. Life span is at least 30, perhaps over 50, years. Maturity is attained as late as 8 years in Great Slave and Great Bear lakes but southern populations mature earlier, as early as age 2 for males and 3 for females. Age at first maturity declines with exploitation as growth rate increases. In Lake Winnipeg, age-groups 5, 6 and 7 accounted for 81% of the catch from 1944 to 1948 but by 1969 88% of the catch was age-groups 3 and 4.
Food
Food is various bottom invertebrates, small fishes and fish eggs although some may feed pelagically on plankton or take terrestrial insects fallen on the water surface. Fishes eaten include Johnny Darters, Spottail Minnows, Ninespine Sticklebacks, Rainbow Smelt, Gaspereau and others. Variation in gill raker number and length in any population indicates the main feeding mode. Lake Whitefish are eaten by Lake Trout, Northern Pike, Muskellunge, Walleye, Burbot, Yellow Perch and others. They eat their own eggs.
Reproduction
Spawning takes place over 2-6 weeks from September to January, earlier in the north than the south, when water temperatures fall below 8°C. Some spawning may occur under ice. Northern populations may only spawn at 2-3 year intervals. The fish enter shallow waters, less than 8 m deep, and release eggs over hard, sandy, silty or weedy bottoms. Some fish spawn in deep water however. The spawning process often takes place at night with much splashing and jumping out of the water. Pairs of fish or 1 female and 2 males rise to the surface and shed eggs and sperm. Yellowish eggs are about 2.6 mm in diameter when shed and may exceed 415,000 per female or 27,460 eggs/kg body weight. The eggs hatch in the following spring, usually April or May, and receive no care from the parents. Larvae are 10.5-11.5 mm long at hatching and live in the top metre of lakes. Post-spawning adults move to overwintering sites, such as the delta in the Mackenzie River. Stocks of this whitefish in different lakes vary considerably in diet, growth rate, movement patterns, fecundity, and egg and larval size.
Importance
This is a very important commercial species in Canadian freshwaters although numbers are declining through overfishing, pollution and the depredations of Sea Lampreys. An annual catch in the Great Lakes has been as high as about 8 million kg, caught with gill nets and trapnets. In western Canada gillnets are set through holes in the ice with a "jigger" which bounces the end of the net from an entry hole to another hole a net's length away. Along with Lake Trout, this species is the principal recreational and commercial fish in northern Canada. The total Canadian catch in 1988 was 9600 tonnes worth about $12 million. Whitefish are marketed dressed, fresh or frozen and in such categories as "jumbo", "large" and "medium". They may also be canned and the eggs made into "golden" caviar. They are considered to be better eating than Lake Trout.
Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1901 the catch was 38,950 lbs (17,683 kg) in the Ottawa River and tributaries, the highest recorded. The Gatineau lakes were the mainstay of the commercial fishery. They also provided winter food for local people, taken on the spawning run in November, while in the summer they were taken by commercial fishermen with nets and spears.
Anglers catch whitefish on hooks baited with a fresh or salted Emerald Shiners or other minnows or by jigging with spoons and colour-beaded hooks. Ice-fishing is popular in the winter.
Cutthroat Trout / Truite fardée
Oncorhynchus clarkii (Richardson, 1836)
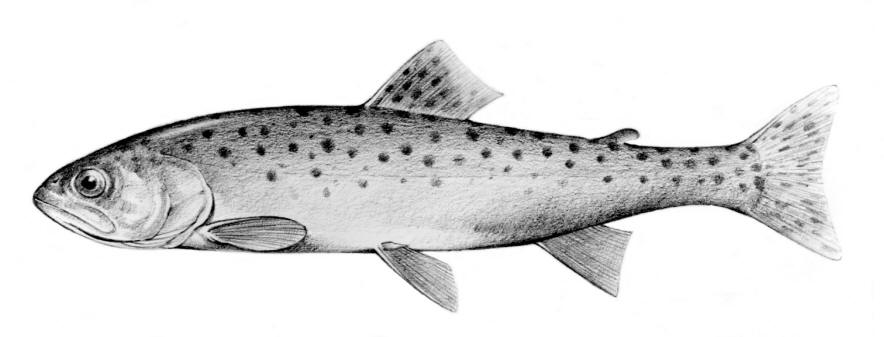

Taxonomy
There are two subspecies of Cutthroat Trout, the Coastal Cutthroat Trout (Truite fardée côtière), Oncorhynchus clarkii clarkii (Richardson, 1836), and the West-slope Cutthroat Trout (Truite fardée du flanc de l'ouest), Oncorhynchus clarkii lewisi Suckley, 1874. The subspecies of specimens introduced to the NCR is unknown. Other common names for the Coastal Cutthroat Trout include Red-throated Trout, Clark's Trout, Sea Trout, Short-tailed Trout, Black- spotted Trout and Harvest Trout and for the West-slope Cutthroat Trout include Red-throated Trout, Lake Trout, Short Tailed Trout, Native Trout, Black-spotted Trout, Montana Blackspot and Yellowstone Cutthroat Trout. This species was formerly placed in the genus Salmo.
Key Characters
The two subspecies are most easily separated by distribution in their native range in western Canada. There is much overlap in their characters and they are difficult to separate. The species itself is identified by the low number of principal anal fin rays (8-12), small dark spots without halos on the whole flank, teeth on the head and shaft of the vomer bone in the roof of the mouth and on the back of the tongue, and the two unique red to orange streaks on the underside of the jaw - the "cut throat".
Description
Dorsal fin with 8-11 principal rays, pectoral rays 12-15 and pelvic rays 9-10. Lateral line scales 116-240 and pyloric caeca 24-60. Males develop a slight kype or hooked lower jaw when in breeding condition.
Colour
Coastal Cutthroat Trout:- Colour is extremely variable and both subspecies have been introduced into the other's range. Hybrids with Rainbow and golden trout further confuse colour patterns and complicate identifications. The "cut throat" marks may be absent in sea dwelling fish or recent migrants to fresh water. These fish have more silvery flanks, are more bluish on the back, have yellowish lower flanks and fins, and spots are less evident. Generally the back is dark green to greenish-blue, the upper flank olive-green and the rest of the flank and belly is silvery. The gill covers are pinkish. Flank spots below the lateral line are more numerous anteriorly (cf. west-slope cutthroat). The outline of spots is irregular, not rounded. Spots are present on the dorsal, adipose and caudal fins and the anal, pectoral and pelvic fin bases. Young have 10 oval, grey-violet parr marks along the lateral line covered with small black dots which extend onto the back and tail. The back may have 5 dark ovals and is olive. The leading edge of the dorsal fin is dark. The dorsal and anal fins may have white patches. The adipose fin has a few black spots. "Cut throat" markings develop on fish over 7.6 cm long.
West-slope Cutthroat Trout:- Colour is very variable and is complicated by the introduction of coastal cutthroat trout. Hybrids with rainbow and golden trout further complicate colour patterns and identifications. The body has a yellow-green to olive back and red on the head sides, anterior flank and belly. The red may be present year round or develop in the breeding season. The flanks are sparsely spotted as are the dorsal, adipose and caudal fins. Flank spots below the lateral line are more numerous posteriorly (cf. coastal cutthroat trout). The flanks may have a narrow pink stripe. The roof of the mouth is whitish in spawning males. Young are the same colour as coastal cutthroat trout. Pure stocks are probably rare in Canada because of introductions from other areas and hybridisation with other trouts such as Rainbow Trout.
Size
Reaches 99.1 cm in non-migrating fish, usually smaller in sea run fish. The world, all-tackle record for a "cutthroat trout" weighed 18.59 kg and was caught in Pyramid Lake, Nevada in 1925.
Distribution
The Coastal Cutthroat Trout is found from southeast Alaska south to California including British Columbia as far inland as the Skeena River headwaters and has been introduced elsewhere. The West-slope Cutthroat Trout is found in western Alberta and southeastern British Columbia and has been introduced into Saskatchewan, Manitoba, Ontario and Québec but perhaps is not established. In the U.S.A., it is found south to Montana (and New Mexico as various subspecies) and has been introduced elsewhere. Bergeron and Brousseau (1981) map this species without an exact locality in the NCR but it is not known if it is maintaining itself (Coad, 1986b).
Origin
An exotic species introduced through a human agency.
Habitat
Coastal Cutthroat Trout are found in fresh and salt waters, migrating between them but not extending into the higher reaches of major rivers like the Columbia in British Columbia. Some populations remain in fresh water. This trout prefers smaller streams or those that have long slow reaches before entering the sea. It is also found in small, coastal bog lakes in British Columbia. Their preferred temperature is <20°C.
West-slope Cutthroat Trout are found in lakes and streams up to over 2440 m, favouring headwaters and small tributaries. Some stream populations are permanent residents, others being spawning fish on a migration from a lake.
Age and Growth
Coastal Cutthroat Trout have a maximum life span of 10 years. Sea run trout are not always larger than stream resident fish because they may not spend long in the sea. Males mature earlier than females, as early as 2 years compared to as late as 6 years. Most fish spawn at 2-4 years.
West-slope Cutthroat Trout have a maximum life span of about 10 years. Males mature earlier than females, as early as 2 years compared to as late as 6 years. Most fish spawn at 2-4 years. In Alberta fish are larger and faster growing in the larger streams and growth is generally faster in warmer water. There are also differences in growth between lake and stream dwelling fish, depending in part on the time spent in streams compared to lake residency.
Food
Coastal Cutthroat Trout food is aquatic and terrestrial insects, crustaceans, small fishes and salmon eggs. Migrating salmon are an important food, taken when cutthroats go to sea at the same time, and other fishes eaten include trout, sculpins, flatfishes, rockfishes and sticklebacks.
West-slope Cutthroat Trout food is aquatic and terrestrial insects, crustaceans, frogs, small fishes and their eggs. Fish eaten include trout, sculpins, carps and sticklebacks.
Reproduction
Coastal Cutthroat Trout spawn in January to May in British Columbia after a migration in late autumn and early winter. There may be late runs in December and January in short streams. Small gravel streams are favoured. The female excavates a redd by lying on her flank and lashing her tail. Redds are about 30 cm across and 10-13 cm deep. Males court females with nudges and by quivering. The female lies in the redd with head and tail bent up, the male joins her, they gape, vibrate and release eggs and sperm. The fertilised eggs fall between the gravel. The female dislodges gravel at the upstream rim of the redd to cover the eggs with up to 20 cm of gravel. The spawning pair may have other males sneaking in to shed sperm. Females may dig more than 1 redd and both sexes spawn with more than 1 other fish. Each female may have 2000 or more orange-red, adhesive eggs of 5.1 mm maximum diameter. The red cut throat may be used in aggressive displays. Sea run cutthroat often survive to spawn again, 12% spawning a fourth time in one study. The fry emerge from the gravel in April and can run to the sea in the spring of their second or third year at about 13-20 cm in British Columbia. They live mostly in estuaries or near shore areas for one or more years, re-entering rivers to spawn in the fall or to feed on migrating salmon in the spring. Growth in the sea can be 25 mm per month. There is variation in migration times, sea life span and spawning time between stocks and geographical areas.
West-slope Cutthroat Trout spawn in spring when water temperatures reach 10°C, June-July in Sheep River, Alberta, May-June in the Flathead River of British Columbia, Alberta and Montana. Small gravel streams are favoured. The female excavates a redd by lying on her flank and lashing her tail. Redds are about 30 cm across and 10-13 cm deep. Males court females with nudges and by quivering. The female lies in the redd with head and tail bent up, the male joins her, they gape, vibrate and release eggs and sperm. The fertilised eggs fall between the gravel. The female dislodges gravel at the upstream rim of the redd to cover the eggs with up to 20 cm of gravel. The spawning pair may have other males sneaking in to shed sperm. Females may dig more than 1 redd and both sexes spawn with more than 1 other fish. Each female may have 2000 or more orange-red, adhesive eggs of 5.1 mm maximum diameter. The red cut throat may be used in aggressive displays. Beach and shoal spawning occurs in some lakes. Fry emerge from the gravel in July-August and stay in streams for up to 4 years before migrating to a lake. Repeat spawning occurs in subsequent years and about 18% of a run is fish which have spawned before. About 7% of the run is non-spawners and 75% are first spawners.
Importance
Coastal Cutthroat Trout are an important sport fish caught on flies, spoons and live bait and often leaps when hooked. In the Bella Coola system, B.C., runs of Chum and Pink salmon in April attract cutthroats which are caught using flies that imitate the salmon fry. Federal and provincial authorities are endeavouring to improve tenfold cutthroat populations in southern British Columbia and Vancouver Island by improving stream conditions where these have deteriorated in populated areas. The flesh is orange-red to pink and best when smoked, fried or baked.
West-slope Cutthroat Trout is an important sport fish caught on flies, spoons and live bait although it does not leap as much as some salmons. The flesh is orange-red and best when smoked, fried or baked.
Rainbow Trout / Truite arc-en-ciel
Oncorhynchus mykiss (Walbaum, 1792)
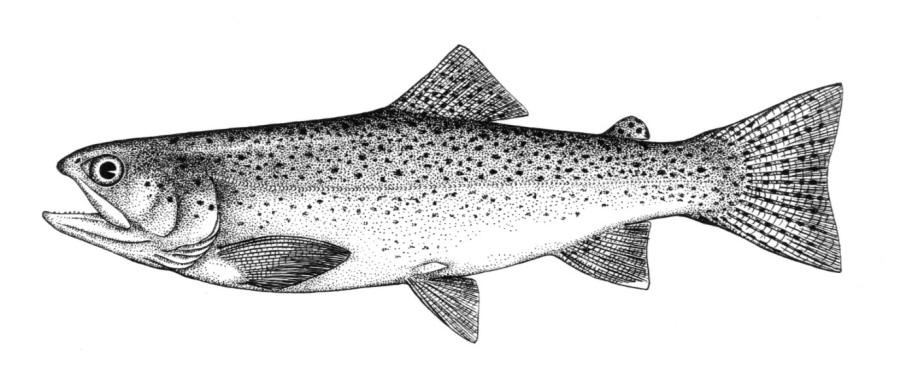



Taxonomy
Other common names include Steelhead Trout, Kamloops Trout, Coast Rainbow Trout, Silver Trout, Half-pounder, Redsides and Pacific Trout. Fish from the upper Columbia, Fraser, Athabasca and Peace River basins are placed in the Redband Rainbow Trout subspecies O. mykiss gairdneri (Richardson, 1836) and those from the coast of British Columbia and the lower Fraser River in the Coastal Rainbow Trout subspecies O. mykiss irideus (Gibbons, 1855). The former subspecies has larger spots, more elliptical parr marks (more rounded in the coastal subspecies), often with additional rows above and below the main parr marks (usually reduced or absent in the coastal subspecies), more yellow and orange tints on the body, a trace of a cut throat mark, and light coloured tips to the dorsal, anal and pelvic fins. The scientific name of the species was formerly Salmo gairdneri.
Key Characters
This species is distinguished by having no red spots on the body but only small dark ones and radiating rows of black spots on the dorsal and caudal fins, no reddish "cutthroat" marks, 100-161 lateral line scales, 8-12 principal anal fin rays, the vomer bone in the roof of the mouth has teeth on its head and shaft, and no teeth at the tongue base.
Description
Dorsal fin principal rays 10-12, pectoral rays 11-17 and pelvic rays 9-10. Pyloric caeca 27-80 and gill rakers 16-22. Breeding males have an elongated snout and the lower jaw is hooked.
Colour
Overall colour is very variable and this is reflected in the common names. Stream fish are darker and more colourful (rainbows) than lighter, silvery lake or sea fish (Kamloops or Steelhead). Some sea-run and lake fish have small orange to red marks below the lower jaw similar to those in Coastal Cutthroat Trout. The back and upper flank are steel-blue, greenish, silvery-olive or even brown, flanks and belly are silvery, grey, white or yellow-green. The side of the head and the flank are characteristically pink. The flank has a broad pink to red or lilac stripe with small black spots. The adipose fin has a black margin and a few spots. Pectoral, pelvic and anal fins may have a few spots and are dusky without any strong markings. Pectoral and pelvic fins are often orange-red. Spawning fish are very dark and the flank stripe is dark red or purple. Breeding males have the roof of the mouth is white. The young have 5-13 dark, oval parr marks centred on the lateral line with the spaces between the marks wider than the marks. There are 5-10 parr marks on the back in front of the dorsal fin. The upper flank has some dark spots. The dorsal fin is tipped white or orange and has a dark leading edge, sometimes broken up into spots. The adipose fin has a black margin. The anal fin has a white or orange tip. Black spots are few to absent on the tail. Some adults in streams do not lose their parr marks. Golden and Palomino trout are genetic variants of Rainbow Trout reared in hatcheries and released into the Great Lakes. Golden Trout (not true Golden Trout, Oncorhynchus aguabonito (Jordan, 1892)) are golden-orange without black spots and Palomino Trout are golden with a few black spots but much less than normal rainbows.
Size
Reaches 122.0 cm and 25.8 kg as sea-run or lake fish but smaller in streams. Possibly reaches 1.5 m and 35.0 kg. Fish less than 10 kg are trophy-size in most of Canada. The 25.8 kg fish was from Jewel Lake, British Columbia. The world, all-tackle angling record from Bell Island, Alaska caught in 1970 weighed 19.1 kg. The Ontario record as of the year 2000 weighed 13.2 kg.
Found in coastal waters and basins draining to the ocean in British Columbia and extreme southwest Yukon and in the upper Peace and Athabasca river basins. Also from Japan and Alaska to Mexico. Widely introduced outside this natural range in all provinces of Canada and world-wide in suitable waters. Broad areas of introduction are southwestern Québec, southern Ontario, all the Great Lakes and across the southern Prairie provinces but also at various places in the Yukon, N.W.T., the Maritimes and in Newfoundland since 1887. This species is known from trout farms in the NCR (Coad, 1986b), and has been stocked in various waters including Meach Lake and Bernard Lake (subsequently disappeared after a 5 lbs (2.3 kg) one was caught in the former lake), the Rideau River above Hog's Back (a half dozen adults from the Central Canada Exhibition, Ottawa in 1935, 1936 and 1937)(Dymond, 1939), and in the Val-des-Bois and other areas of Québec (Finn, 1964; Dumont et al., 1988). None were caught in a survey of the Rideau River between 1998-2000 (www.rideauvalley.on.ca/programs/rrr/rrr.html, downloaded 15 July 2002). It has been suggested that it could be successfully stocked in the Ottawa River at Ottawa (Hopkins, 1996a).
Origin
A species introduced by man to the NCR.
Habitat
Rainbows are found in rivers or streams where there are pools and riffles. Some live in lakes and are called Kamloops Trout while others run to sea and are called Steelheads in their natural habitat. They can tolerate temperatures up to 24°C, warm for a trout, but prefer temperatures below 20°C and their preferred temperature is 11.3°C. The Great Lakes have introduced Steelhead stocks. Lake populations run up streams to spawn while river fish enter headwater streams.
Age and Growth
Life span varies with habitat, up to 11 years in some lake fish, 8 years in the Great Lakes but only 3-4 years in many streams and small lakes. Growth varies with habitat including such factors as length of sea, stream or lake life, years before spawning, available food supplies, latitude, altitude, temperature regime, competition with other salmonids, and so on. Ageing these fish may be difficult because of the complicated life history pattern of stream and lake residency. Maturity is also variable with habitat. Some males mature at 9 months in fish introduced to warm southern waters and some females only at 8 years, but generally maturity is reached at 3-5 years in Canada with males maturing a year earlier than females.
Food
Food includes plankton, crustaceans, aquatic and terrestrial insects, snails, leeches, worms, molluscs, salmon eggs, and many fishes. The fish eaten enhance growth and the species taken depends on what is available. Rainbows are food for various other Salmon Family members found in the same habitat, most predatory fishes, as well as various birds and some mammals. Lampreys attack this trout.
Reproduction
Repeat spawning can occur for up to 5 years and in the NCR this can occur in streams and lakes. Spawning takes place from March to August but is usually in spring. Great Lakes fish may spawn from late December to late April. Water temperature for spawning usually exceeds 10°C but may be 5-13°C. A female excavates a redd by lying on her side and thrashing her tail. Redd excavation occurs during the day and night and dimensions are usually longer and deeper than the female's body. Females construct several redds and may spawn with several males. A male courts a female by rubbing his snout and body against her, by vibrating, by swimming over her in the redd and by pressing against her. Several males are found around each female but one male is dominant. The spawning act lasts 5-8 seconds with the pair parallel in the redd pressed together, both fish gape, arch and vibrate. Other males may shed sperm. The female covers the eggs with gravel by dislodging it from the upstream end of the redd. Most spawning takes place in the morning and evening and nests may be abandoned during the day. Eggs are orange or pink, 5.0 mm in diameter and up to 12,749 in number. Egg numbers are usually a few hundreds to thousands. The eggs hatch in about 8 weeks and fry generally emerge in June to August from spring spawnings.
Importance
Rainbow Trout are one of the top few sport fishes in North America and of great commercial importance because of the money spent on gear, accommodation, transport, etc. by anglers in pursuit of this fish. Many books and articles have been written on the methods and joy of catching this trout. It may be caught on flies, with lures or with various still-fished or moving baits. It is a strong fighter which bites easily and leaps often, and may "cartwheel" and "tail-walk". Dawn and dusk are the best times to catch rainbows in streams. Trolling an artificial fly is used on large lakes. Baits and small jigs are used in ice fishing. The flesh is excellent eating fresh or smoked and may be red if food is mostly invertebrates or white if food is fishes. The total Canadian catch in 1988 was 3658 tonnes. Farmed fish are sold frozen world-wide, the most important trout in this regard, and ironically some of that found in Canada comes from Japan or Europe. Small put-and-take fisheries are common wherever this species is found or has been introduced. Rainbows have been used extensively as research animals as their requirements are well known and they are readily available from hatcheries. Hatchery fish often have reduced or absent fins and deformed mouths. Introductions of this fish to various waters throughout Canada may have serious consequences for other species.
Atlantic Salmon / Saumon Atlantique
Salmo salar Linnaeus, 1758
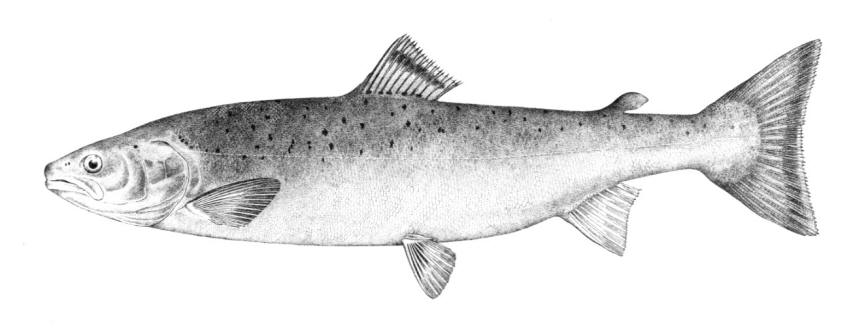


Taxonomy
Other common names include Saumon de l'atlantique, Ouananiche, Landlocked Salmon, Lake Atlantic Salmon, Sebago, Kennebec Salmon, Black Salmon, Grayling, Bratan, Saumon d'eau douce, Sâma. Saama, Saamakutaak and Kavisilik.
Key Characters
This species is characterised by having 109-124 lateral line scales, 10-12 principal dorsal fin rays, 8-11 principal anal rays, vomer bone in the roof of the mouth with teeth on its head and shaft, no spots or rows of spots on the caudal fin, 2-3 large spots on the gill cover, upper jaw not usually extending beyond the eye, no red on the flank or adipose fin in adults.
Description
Pectoral rays 14-15 and pelvic rays 9-10. Pyloric caeca 40-74. Spawning males develop a kype, or hooked lower jaw.
Colour
The back is brown, green or blue, flanks are silvery, and the belly is white. Black spots, often x- or y-shaped are found on the upper flank and back and sometimes but not usually on the caudal fin. Spawning males have bronze to dark brown colour and may have red, orange or rust-brown spots on the head and body. The pectoral and caudal fins may darken. Spawning females are grey to purple-blue and may become blackish. After spawning they become very dark and are known as kelts, slinks or black salmon. Young fish have a red spot between each of 8-11 narrow, parr marks. Young fish migrating to sea, known as smolts, racers, grilts or fiddlers, become silvery. Ouananiche have dark brown, bronze or bluish flanks with larger spots than sea-run fish and sometimes light halos around the spots.
Size
Reaches 184.0 cm and 46.8 kg, possibly reaching 2.0 m. The world, all-tackle angling record from Norway in 1928 weighed 35.89 kg. The Ontario record as of the year 2000 weighed 11.0 kg. The land-locked salmon (Ouananiche) angling record is 10.31 kg for Lake Lobstick, Newfoundland in 1982.
Found from Ungava Bay south to the Gulf of Maine throughout Atlantic Canada including the Grand Bank and over deep ocean east of the Grand Bank, and up the St. Lawrence River, once to Lake Ontario. Rare specimens enter eastern Hudson Bay. Across the Atlantic to Greenland, Iceland and in western Europe. There are a number of naturally land-locked populations, known as Ouananiche. Blais and Legendre (1978) give several localities in Québec where Ouananiche and Atlantic Salmon have been introduced, many close to the NCR, but continued survival is unknown (Coad, 1986b). Only one locality falls within the NCR, at Lac St-Charles east of Brennan Hills and the Gatineau River (45°46'30"N, 75°52'25"E) where 1000 Atlantic Salmon alevins were introduced in 1966 by the Club Byng. Ouananiche were also stocked in Lac Deschênes of the Ottawa River on 31 October 1989, numbering 400 2+ age fish but none were ever caught (Chabot and Caron, 1996). Also introduced to Ontario, Alberta and British Columbia.
It remains uncertain that salmon ever ascended the Ottawa River as far as the NCR. Van Cortlandt (1865b) states "this splendid fish never visits the Ottawa waters, nor, as far as I can learn, ever has done so." He later states "That the true Salmon, Salmon Salar, was wont to visit our tributaries, and this too within a very limited period, does not admit of the shadow of a doubt" (Van Cortlandt, 1867; reprinted 1999, possibly accounting for the spelling "Salmon Salar"). Small (1883) comments that there is a lack of any authentic data that the salmon was ever abundant in the Ottawa River prior to sawdust and mill refuse pollution. This implies, but does not confirm, a presence. He then states that the name Salmon River near Montebello "was retained ..... long after the native fish had abandoned its waters", again implying a presence but without clear confirmation. Early attempts at stocking the Salmon River at Montebello failed. There was a hatchery in Ottawa founded in 1890 that produced Atlantic Salmon (Rodd, 1912) but whether these were ever stocked locally was not stated therein. Egan (1999) mentions salmon making it up the Ottawa as an attraction for settlement of Hull by Philemon Wright in 1800 and Egan (1996b) mentions a run reported by early settlers up to the Chaudière Falls. Salmon (assumed to be Atlantic Salmon but possibly a Pacific species introduced to the the Great Lakes) have been caught at Hawkesbury below the Carillon Dam. If they are Atlantic Salmon then they are presumably strays from a stocking programme or migrants from the Atlantic Ocean (Egan, 1996a; 1996b; 1997).
Origin
This species has its origin in an Atlantic refugium.
Habitat
Atlantic Salmon adults are found in coastal waters and some travel as far as Greenland, a migration of about 2400 km, or even Norway. They home to their birth river to reproduce after 1 or more years at sea. The ability of salmon to home to their birth stream over thousands of kilometres has not been fully explained. The earth's magnetic field, ocean currents or stars may be used to navigate to coastal waters where smell directs them to their stream of birth. Fish returning to freshwater after 1 year are known as grilse and weigh 1.4-2.7 kg. Fish with 2 sea years weigh 2.7-6.8 kg and are known as salmon. Ouananiche are generally smaller than fish which have access to the rich food in the sea. Ouananiche in Gros Morne National Park, Newfoundland have slow growth, a small maximum fork length of 25 cm, low fecundity at a maximum of 268 eggs, and retain juvenile characters. Young salmon or parr spend 2-4 years in fresh water in cool streams and rivers until 12-15 cm long when they run to sea as smolts. In Ungava this is delayed until 4-8 years of age and 18 cm. They may spend 6 or more years in the sea. Landlocked salmon remain in a lake and run into tributaries to spawn. Some Ouananiche may have access to the sea but do not migrate while others are truly landlocked by physical barriers. Males grow faster and are larger than females. Their preferred temperature is 16°C.
Age and Growth
Maximum life span is 11 years as some fish survive spawning and run to sea more than once. Salmon in the sea grow much more rapidly than those in freshwater because of the year-round access to a wide range of foods compared to a 4-month feeding period restricted to smaller, less varied and sparse aquatic insect supplies. Maturity is attained at age 3. Fingerlings of Ouananiche (landlocked Atlantic Salmon) have been introduced to Papin Lake in Pontiac County (76°06'N, 46°09'W) just outside the NCR from 1961 to 1973 (Cuerrier, 1983). Growth was rapid as Pygmy Smelt (Osmerus spectrum) had also been introduced as a forage fish. After three summers some salmon had attained 2.0-2.84 kg with sexual maturity at age 2+. The largest fish caught was over 9.0 kg and the oldest was 8 years. There was no natural reproduction in the absence of suitable spawning streams and salmon are no longer present.
Food
Food of young salmon in freshwater is aquatic and terrestrial insects, crustaceans and fishes and of adults in the sea various crustaceans and a wide variety of fishes such as Capelin, Rainbow Smelt, Gaspereau, Atlantic Herring, Atlantic Cod, sand lances and mackerel. Salmon in lakes prefer Rainbow Smelt. Salmon on the spawning run do not feed and why they readily strike at wet and dry flies is something of a mystery. American Eels, sharks, Swordfish, Pollock, tuna, birds and seals all eat salmon as young or adults.
Reproduction
Although some fish spawn twice, very few survive the arduous migrations and spawning acts to reproduce for a third time. Rarely some fish spawn 8 times. Repeat spawners may comprise as much as 34% of the Grand Cascapedia River, Québec population while in other rivers repeat spawners are as low as 5%. Salmon spawn in October and November after entering estuaries from spring to autumn. Ouananiche in the Outaouias spawn too in October and November at water temperatures around 5C (Thellen, 1994). The time of entry to each river is the same each year. The timing of entry depends in part on distance to travel. Spring runners often run to distant headwater streams and fall runners to lower reaches. The migration is not blocked by rapids or low waterfalls which the salmon leaps spectacularly, sometimes over 3.5 m. The female excavates a redd in gravel bars and riffles by turning on her side and lashing her tail. Redds are up to 5.9 m long and 0.9 m wide and are found in water about 25 cm deep. Non-anadromous salmon in Newfoundland scatter their eggs among boulders while the anadromous salmon in the same stream construct redds. The male drives away competitors particularly grilse. Large male reproductive success depends on successful competition for access to a female as the large male rarely survives spawning. The female enters the redd by backing in and tests it with her anal fin for spawning suitability. She is joined by the male and eggs and sperm are shed as the pair quiver and gape. The male may nudge the female and glide across her. The female uses her tail at the upstream end of the redd to dislodge gravel and bury the eggs as deep as 25.4 cm. Small male parr, only 10-15 cm length, rush in to fertilise eggs during the spawning act. These are known as "sneaky males". In some Canadian waters, such as the Matamek River, Québec, the proportion of grilse has increased from about 70% to about 85-90% and precocious parr (sneaky males) are much more numerous. The capture of large adults at sea by commercial fishermen has shifted reproduction to earlier life history stages. The shift is genetic as grilse matings result in offspring which return to spawn as grilse rather than salmon. The large adults repeat the redd excavation and spawning for a week or more. Eggs are up to 7.0 mm in diameter, orange to amber and number up to 629 per kilogram of female body weight (and some females have over 20,000 eggs). Spawned out fish or kelts may rest in a pool for a few weeks or over winter or run to sea immediately. Eggs hatch in April after overwintering and the young fish, known as alevins, emerge from the gravel in May and June. Small salmon are called fingerlings or underyearlings and parr once the characteristic flank marks develop. Parr retreat into the gravel and under large rocks in the fall when temperatures drop to 9-10°C and re-appear only in spring and early summer when waters warm up. During the summer, most parr are found above the stream bed holding station over a stone.
Importance
Atlantic Salmon are the quintessential sport fish as well as having great commercial importance. Comments on the lore and techniques of catching salmon are superfluous as numerous books have been written. They are easy to catch on the spawning migration using flies. Salmon rivers are a valuable commercial resource, access being controlled by public and private clubs. Their concentration in rivers for spawning make them susceptible to environmental changes and their high seas life to commercial exploitation by nations without an interest in conserving freshwater runs. The coastal fishery in Newfoundland and Labrador for example includes some fish from other Canadian provinces and from Maine. The West Greenland area is very important for maintaining Canadian stocks which are dependent on limitations on this fishery. About 40% of Canadian fish visit the Greenland feeding grounds and some travel as far as the Faroes. The annual North Atlantic catch is about 10,000 tons or 3 million fish. The Canadian catch in 1988 was 3847 tonnes and landings were worth about $4 million. Lake Ontario salmon were fished to extinction by 1890, compounded by construction of dams which blocked spawning streams. In addition to dams throughout its range, various pollutants and acid rain are just a few of many threats to this species. The species is now being farmed in Canada with cage-reared fish in the Bay of Fundy being worth an estimated $16 million in 1987, with figures for more recent years expected to reach about $100 million. Attempts are being made to incorporate the "anti-freeze genes" from winter flounder into salmon to facilitate winter survival in inshore waters and so make cage-culture more effective.
Brown Trout / Truite brune
Salmo trutta Linnaeus, 1758
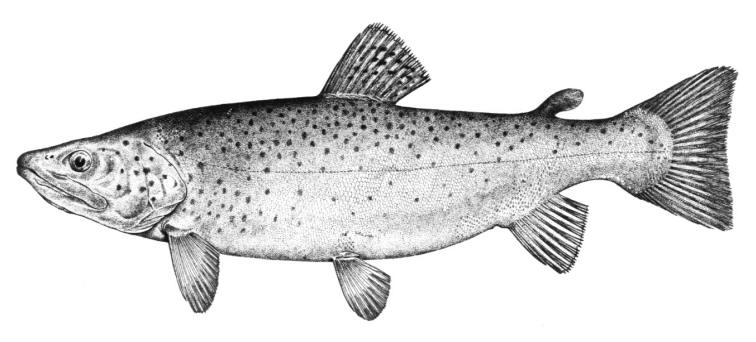

Taxonomy
Other common names include German Brown Trout, European Brown Trout, Sea Trout, Brownie, Loch Leven Trout, Von Behr Trout, Spotted Trout and Liberty Trout.
Key Characters
This species is a relative of the Atlantic Salmon and is distinguished from it by the upper jaw extending beyond the eye in adults (and below rear half of the eye in young rather than the centre in young), the gill cover has many spots, dorsal fin principal rays usually 10-14, and orange to rusty-red spots are often present on adult flanks.
Description
Principal anal fin rays 9-12, pectoral rays 13-14 and pelvic rays 9-10. Lateral line scales 110-136 and pyloric caeca 30-60. Males develop a hooked lower jaw when spawning and the brown colour becomes more intense and golden.
Colour
Overall colour is a light to golden brown with silvery flanks and a white to yellowish belly. The back, flanks, side of the head and dorsal and adipose fins bear black or dark brown spots, often with a lighter halo of orange, pink or red, and the flank has pink or red spots. Only Brown Trout have both light and black spots on the flanks. The caudal fin may have spots restricted to its upper lobe but lacks the overall radiating spots of Rainbow Trout. The adipose fin is orange to orange-red, the only family member with this colouration. Sea run or lake fish are more silvery, obscuring some of the spots. Spots may be x- or y-shaped. The red flank spots usually have blue halos. Young have 7-14, narrow parr marks and a few red spots along the lateral line. The adipose fin is orange with a light margin. Hybrids with Brook Trout are called Tiger Trout for their distinctive markings
Size
Reaches 1.4 m and reputedly 50 kg. The world, all-tackle angling record from Arkansas in 1992 weighed 18.25 kg. The Ontario record as of the year 2000 weighed 15.6 kg. Brown Trout over 2 kg and 16-24 inches long have been caught at Remic Rapids in Ottawa from smaller stocked fish (see below).
Found in all Canadian provinces, except P.E.I., Yukon, Nunavut and Northwest Territories, as an introduced species from Europe and western Asia. Most widely distributed in southern Ontario and Alberta, sporadically elsewhere.
In the NCR, this species has been introduced to the Mississippi River (Anonymous, 1932; Dymond, 1939; the latter noting that none survived there to be caught by anglers despite numbers of 15,000 in 1931, 25,000 in 1932 and 10,000 in 1934 and 1500 15 month old fish in 1937); the Rideau River below Hog's Back in 1937 (1600 yearlings) and above Hog's Back (a half dozen adults from the Central Canada Exhibition in Ottawa); in waters of the Seigniory Club near Montebello, Québec (Dymond, 1939); 3600 in the Rideau River at Mooney's Bay just above Hog's Back in 1941, many eaten by gulls at low water the following spring (newspaper reports; Bebee, 2004); east of Hull (Dumont et al., 1988); the Kemptville area (Lasenby and Kerr, 2001); and the Remic Rapids in the Ottawa River in the late 1980s, in 1997 (15,000 three-inch fish (Hopkins (1997a; c)) and again in 1999-2000 (5000 fish mostly at 15-20 cm and some adults at 50 cm(Hopkins, 2000)) or in another report 10,000 7 inch fish, 500 10 inch fish and 120 fish between 2.5 and 5.5 lbs (www.flyanglersonline.com/features/canada/can131.html, downloaded 3 June 2003). This latter source reports that it was the Province of Québec that stocked rapids west of Ottawa in 1987 with 14,000 fingerlings and 11,000 fingerlings and 20,000 7 inch fish between 1990 and 1994. Chabot and Caron (1996) summarise stocking reports from the Deschênes Rapids which presumably includes "rapids west of Ottawa" mentioned above. They give 14,000 fingerlings in 1987, 5000 1+ age young on 14 May 1990, 10,000 fingerlings on 17 October 1990 (Hopkins (1991b) has 15,000 fish in 1990, most fingerlings but 5000 one-year-olds and 100 two-year-olds), 100 2+ fish on 30 November 199? (date incomplete, ?1991), 5000 1+ fish on 30 April 1992, 5000 1+ fish on 22 April 1993, 5000 1+ fish on 3 May 1994 and 7500 fingerlings on 27 October 1994, as well as 5000 1+ fish at Kettle Island in 1992.
None were caught in a survey of the Rideau River between 1998-2000 (www.rideauvalley.on.ca/programs/rrr/rrr.html, downloaded 15 July 2002) but some are caught in the Ottawa River near Ottawa still.
Several local creeks were proposed as stocking sites for brown trout, namely Findlay or Kelly near Leitrim, Poole in Stittsville and Shield near Osgoode (Hopkins, 1993d) but this may never have happened.
Origin
This species is introduced by man to the NCR.
Habitat
Brown Trout are mostly stream and river dwellers although some are in lakes and ponds. They can tolerate warmer and more turbid waters than Brook Trout and only the Rainbow Trout is more tolerant among salmonids. Brown Trout may survive in areas no longer suitable for Brook Trout because deforestation has increased stream temperatures and agriculture and industry have increased pollution and turbidity. Ideally there should be overhanging and submerged vegetation, coarse gravel and cover such as logs, boulders and undercut banks, growth is better in alkaline waters (20-200 mg L-1, total dissolved solids 800 mg L-1 for culturing this species), a depth of 7-58 cm is needed for spawning, a winter flow of less than 15 cm sec-1 is required, preferred temperatures are 10.0-17.6°C (other reports give 21.1°C and temperatures up to 24°C are tolerated) and for spawning 6.7-8.9°C, dissolved oxygen is optimal at 7-9 mg L-1 and pH range tolerated is 5.0-9.5 although the optimal range is 6.8-7.8.
Age and Growth
Life span is over 18 years with maturity attained at 2-4 years.
Food
Food is aquatic and terrestrial insects, crustaceans, molluscs, various fishes, frogs, salamanders and even small mammals. Most food is taken as drift, the trout positioned in slower water behind a rock and darting out into faster current to seize prey. Predators include other fishes, birds, water-snakes and otters.
Reproduction
Spawning occurs from October to January depending on locality, usually at 7-9°C but as low as 2°C or as high as 14°C. In the Outaouais, reproduction occurs in the second half of October and in November (Thellen, 1994). It usually takes place in gravel stream riffles but may occur on rocky shores of lakes. The female excavates a redd into which the spawning pair deposit eggs and sperm while gaping and quivering over a 4 second period. The female covers the redd with gravel after spawning to protect the eggs. Subsequent spawning occurs, usually after a 10 hour interval. Occasionally a community redd is excavated by a number of fish spawning close together. Eggs are up to 5.0 mm in diameter and each female can contain 20,865. Young may spend about 2 years in their natal stream before going to a lake but some fish remain permanently in streams.
Importance
This trout is a valuable and popular sport fish in Europe, less so in Canada because of its localised introductions. The benefits of stocking Brown Trout include its difficulty of capture (and thus appeal to anglers), its longer contribution to the fishery than Brook Trout and Rainbow Trout, better survival and growth than other trouts, and its tolerance of warmer waters. There is an extensive European literature on angling methods and it is reputed to be a wilier fish than native Brook Trout in Canada. However studies in an Ontario stream compared catchability favourably with native species. They tend to bite best in the late evening or at dawn, especially when large. These trout are caught on worms, crayfish, lures and flies. Brown Trout have white to pink flaky flesh and are excellent eating. Flesh colour changes with age, to pink, and is related to diet.
Arctic Char / Omble chevalier
Salvelinus alpinus (Linnaeus, 1758)
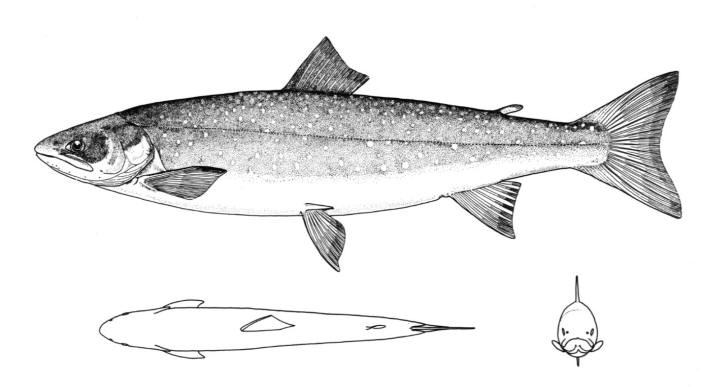
Taxonomy
Other common names include Alpine Char, Silver Char, Hearne's Salmon, Sea Trout, Coppermine River Salmon, European Char, Hudson Bay Salmon, Mountain Char, Arctic Salmon, Blueback Trout, Greenland Char, Québec Red Trout, Omble rouge du Québec, Truite rouge du Québec, Ekaluk, Ilkalupik, Kaloarpok, Ivatarak, Iloraq, Aniaq, Nutidilik, and many others. Char may be spelled charr.
There is considerable variation in characters of Arctic Char around the Northern Hemisphere and even within one lake. The species is known as the Arctic Char "complex" as an acknowledgement that more than one species or subspecies may exist. Landlocked forms in Québec were known as Québec Red Trout or Marston Trout and was given the scientific names marstoni. They are generally recognised now as relict Arctic Char, descendants of an older, anadromous form of char which became landlocked and isolated from later char forms invading eastern North America. An alternative view is that char forms are the result of generalist or specialist life history styles, the former in perturbed environments and the latter in unperturbed environments.
Key Characters
This species is characterised by having small scales (123-152 in the lateral line), few major anal fin rays (8-11, total 11-15), teeth only on the anterior or head end of the vomer bone in the roof of the mouth, a truncate or square-cut caudal fin, and colour pattern.
Description
Teeth are present on the jaws, tongue and the palatine and basibranchial bones. Major dorsal fin rays 10-12 (total 12-16), pectoral rays 14-16 and pelvic rays 9-11. Pyloric caeca 20-75, upper arch gill rakers 7-13, lower arch rakers 12-19. Males develop a kype or hooked lower jaw during spawning in anadromous populations. Anadromous males also develop a dorsal ridge anterior to the dorsal fin and enlarged teeth, characters not found or only poorly-developed in landlocked males.
Colour
Colour varies between landlocked, migratory and spawning fish and between individuals by sex. However Arctic Char lack spots or vermiculations on the dorsal and caudal fins found in its relatives, the Lake Trout and Brook Trout. The pectoral, pelvic and anal fins have a white leading edge, followed by contrasting black and red bands, with the rest of these fins and other fins dusky. Generally non-spawners are an overall silvery colour. A spawning male has a brown, dark green to blue-green or steel blue back, flanks are silvery-blue with white to cream, orange to pink or red spots larger than the pupil and the belly is white or golden to a bright orange-red. Some populations lack spots while in others the spots have a blue halo. This bright spawning colour may be retained year-round in some freshwater populations. Isolated, stream-resident males retain parr marks, have yellow flank spots and are nearly black dorsally. Young Arctic Char have 8-17 oval parr marks along the flank.
Size
Reaches 108.0 cm and 20.0 kg. The world, all-tackle angling record from the Tree River, N.W.T. weighed 14.77 kg and was caught on 30 July 1981 by Jeffrey L. Ward.
Found around the Northern Hemisphere including the Arctic coast and islands of Canada, Labrador, and northern Québec, Newfoundland, the Québec north shore and in lakes of southwestern Québec and New Brunswick. Introduced to Alberta. Also in lakes of Maine and New Hampshire in North America. In the NCR they may no longer be present in many of the lakes mapped through overfishing and competition with introduced Smallmouth Bass.
Origin
This char survived in an Atlantic coastal refugium or a Beringian refugium (Mandrak and Crossman, 1992).
Habitat
In the NCR char are found in deep and cool waters of lakes during the summer months. They may approach the surface or shallow water during the cooler days of spring and fall. Elsewhere they may be found in streams and rivers.
Age and Growth
Life span exceeds 40 years and growth varies between localities. Isolated stream resident fish seldom live longer than 10 years however. Landlocked fish tend to be smaller than searun fish of the same age. In Candlestick Pond, Newfoundland landlocked char have slow growth, a short life span of 7 years, small size (to 16.4 cm fork length), maturity at age 3 and low fecundity (104 ova per fish maximum). Maturity can be attained at 1 year for landlocked females in Newfoundland but anadromous fish can mature as late as 10-25 years with ages in between for various populations.
Food
Arctic Char are predators on various crustaceans, insects, snails, clams and fishes, feeding in both salt and fresh water on whatever is available. Amphipods are an important food when at sea near Baffin Island. In a Newfoundland lake Rainbow Smelt were the predominant food. Diet shifts occur depending on which, if any, fish species the char is sympatric with. They are cannibals and are eaten by some birds and seals.
Reproduction
Spawning occurs in August to October in the far north or as late as December-January in southern Québec. Char which spawn do not migrate to the sea but may go downriver as far as estuaries. Females spawn only every 2-3 years in the north but may do so annually in the south. The absence of a sea migration in northern populations which spawn is a major energy drain and prevents fish from spawning 2 years in succession. In the central Canadian Arctic and Arctic islands spawning takes place in lakes as the rivers are completely frozen in winter. Females clear a nest area by turning on their sides and flapping the caudal fin on stream gravel or rocky lake shoals. Pair spawning takes place at about 4°C during the day. Males guard territories and may spawn with more than 1 female. The male circles the female on the redd, quivering as he passes along her flank. The female and male quiver and release the sex products during one of the passes by the male. The female undulates over the eggs to force them into the gravel and dislodges upstream gravel to cover the eggs but only after several spawning acts. A female may construct 10 or more redds but is eventually abandoned by the male when she is spawned out after 4 hours to 3 days. The male will court a new female. Eggs are up to 5.5 mm in diameter when deposited and number up to 9245. The eggs hatch at ice break-up in the following spring. Temperatures over 7.8°C will kill the eggs.
Importance
Arctic Char have long been used as food for humans and for dogs in the north and are caught in nets, traps and by spearing. They are eaten fresh, smoked, salted or dried. The flesh is red, pink or white. The 1988 Canadian catch was 88 tonnes valued at $486,000. They are strong fighters and sought after by anglers. Smaller fish may leap though large ones seldom do so. Char are caught on streamers, spoons and dry flies, which they may follow for long distances before striking. Char populations grow slowly and have a low fecundity so they can easily be fished out. However some char are now being farmed in fresh and salt waters. Char are important research tools for biologists, being the only fish species present in some undisturbed northern lakes. They can be used to test conceptual models since the ecosystem is much less complex than the multi-species lakes of southern Canada.
Brook Trout / Omble de fontaine
Salvelinus fontinalis (Mitchill, 1814)
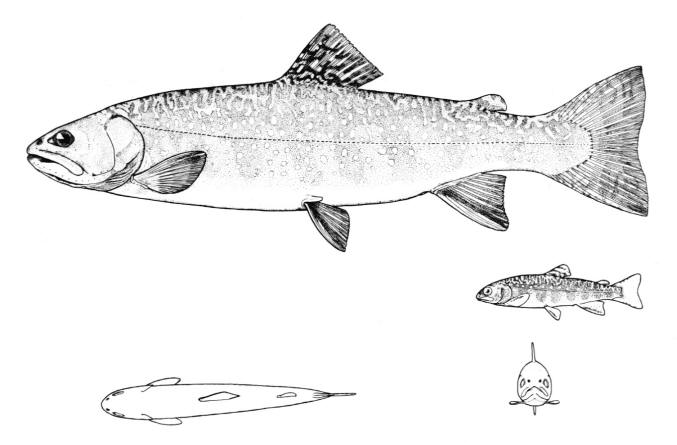


Taxonomy
Other common names include Brook Char(r), Speckled Trout, Eastern Brook Trout, Brookie, Square-tail, Sea Trout, Salter, Redspotted Trout, Mud Trout, Slob, Coaster, Harness Trout, Native Trout, Mountain Trout, Speck, Whitefin, Truite mouchetée, Truite de mer, Truite de ruisseau, Truite saumonée, Iqaluk Tasirsiutik, Anokik, Anuk, Aanak, Anakleq, Aanaatlik, A Na, I Ha Luk and Âna.
Key Characters
This species is characterised by 109-132 lateral line scales, 8-13 anal fin principal rays, teeth on the head of the vomer bone in the roof of the mouth, truncate caudal fin, pectoral, pelvic and anal fins with a white leading edge followed by contrasting black, red spots ringed with blue on the dark body, dorsal and caudal fins have wavy, dark lines and blotches and the back has dark or light green or cream, worm-track markings (vermiculations).
Description
Dorsal fin principal rays 9-14, pectoral rays 10-15 and pelvic rays 7-10. Pyloric caeca 20-55. Spawning males develop a hooked lower jaw or kype and have larger pectoral and anal fins than females and longer upper jaws.
Colour
The back is olive-green to dark brown or blackish fading to a silvery-white belly. Flanks have a red to yellow tint. Flank spots are pale but there are also small, red spots with blue halos. The pectoral, pelvic and anal fins are yellow, orange, or reddish behind the white and black leading edges. Trout in large lakes are also more silvery than stream resident fish. The jaw tips and the roof of the mouth are blackish. Spawning males are much brighter in overall colour and have an orange-red lower flank and upper belly, bordered below by black on each side which delimits the white belly. Young have 6-12 brown parr marks, the widest equal to eye diameter, and small red, yellow or blue flank spots. The white leading edge to the lower fins is apparent.
Size
Attains a reputed 90.0 cm and 8.0 kg, possibly 9.39 kg, but most are smaller. The world, all-tackle angling record was caught in the Nipigon River, Ontario in July 1916 by W.J. Cook and weighed 6.57 kg.
Found from the shores of Hudson Bay and Labrador south in marine waters to Cape Cod in the east and Georgia in the Appalachian Mountains, west through all the Maritime provinces, Québec and Ontario (except the extreme west) to northeast Manitoba. Widely introduced to western Canadian provinces, the U.S.A., South America, Europe, Asia and Australasia. In the NCR, it is almost entirely restricted to the Québec side although there are some surviving in Kelly's Creek in Ontario. Hopkins (1993c) records trout in the past from Poole Creek in Stittsville but none in a September 1993 survey despite 500 being stocked in the spring, presumably because of warm summer temperatures. Findlay (= Kelly's) Creek in Leitrim was stocked in spring 1993 too. Hopkins (1993c) also reports trout formerly in Shields Creek at Greely. The disappearance of Brook Trout in Gatineau Park lakes is attributed to the introduction of Smallmouth Bass which both ate them and competed with them for the same foods (Rubec, 1972; 1975). Rubec (1975) lists 15 lakes having this species in the late 1800s in Gatineau Park and only 5 of these still retain populations at the time of his survey. Brook Trout have been widely introduced into smaller lakes in Gatineau Park, such as Black Lake which provided sport for anglers in 1972 and 1973, while those introduced to Lusk Lake had limited reproduction (Rubec, 1972).
Origin
Brook Trout could have entered the NCR from either an Atlantic or Mississippian refugium or perhaps both. Mandrak and Crossman (1992) refer to an Atlantic refugium origin.
Habitat
Brook Trout are found in cool waters of streams and lakes, usually at less than 20°C. Their preferred temperature is 16.0°C. Pools, underneath banks, under overhanging bushes or behind rocks are favoured spots. Most specimens from the NCR were captured over stony or gravel bottoms with slow to medium currents. During summer months they retreat to deeper water, to about 8 m, in lakes. Populations in the Great Lakes live and feed mostly in the lake and run up natal streams to spawn. They are known as "coasters".
Age and Growth
Maximum life span is over 20 years but most reach only 5 years. Maturity is attained at 2-3 years, some males at 1 year. Growth is often faster than other trouts and chars. Stunting is common in small streams while sea run fish grow faster than freshwater ones. Optimum growth temperatures are 10-19°C.
Food
Food includes aquatic and terrestrial insects, molluscs, crustaceans, worms, various fishes, frogs, salamanders and even snakes, mice, voles and shrews. Stream-dwelling fish feed heavily on drifting aquatic organisms during spring run-off but in summer as drift decreases surface insects become important. Most feeding occurs in the early morning and late evening although some food is taken throughout the day. Diet shifts in response to competition with other species. Trout feed on large, bottom invertebrates in some Québec lakes but switch to zooplankton when found with Creek Chub. Trout are more aggressive in groups but chub forage successfully in groups. Sea run fish take various marine invertebrates and fishes. Sea run adults in spring and summer eat crustaceans and fish in lower estuarine areas while young are in the upper estuary eating crustaceans and insects. During the fall in the river adults eat little and during winter back in the estuary consumed mostly crustaceans. There is thus a division of food resources between young and adults. Brook Trout are cannibals on their eggs and young and are eaten themselves by other fishes, water snakes, turtles, various birds and otters.
Reproduction
Spawning takes place from August to December, earlier in the north and later in the south. Sea-run trout enter their natal stream in spring and summer even though spawning occurs in fall. Each year they spend 1.5-3 months feeding in the sea. The spawning ground is usually gravelly streams but may be lake shoals if there is some current or spring outflow to keep eggs oxygenated. Spring flows are preferred even in streams. Males arrive on the spawning ground first and defend a territory. Both sexes will rush at other fish entering the redd area. The female cleans a redd of debris by turning on her side and lashing her tail. Redd depth between stones is tested by inserting the anal fin. Redd construction may take up to 2 days with work being carried out both by day and night. Courtship involves gentle pushes, touches and strokes of the female by the male. The female is ready to spawn when she crouches in the redd with her genital area between the stones. The male arches his body and may press the female against the redd bottom, both fish vibrate and eggs and sperm are shed. Accessory or sneaky males may rush in to shed sperm. The female lashes her tail to push eggs into the gravel and then dislodges gravel with her anal fin to cover the eggs to depths as great as 20 cm. Yellow-orange eggs are up to 5.0 mm in diameter and number up to perhaps 17,000 per female although averages range from the low hundreds to a few thousand. Both sexes may spawn again with other fish. The eggs develop over winter, taking 165 days at 2.8°C but only 47 days at 10°C. Temperatures above 11°C will kill the eggs.
Importance
Brook Trout are very popular sport fish in eastern Canada caught on lures, live baits and flies. These trout are easier to catch than Brown Trout and take a wider range of lures. They fight well but are often quite small and do not leap spectacularly like some Salmon Family members do. Trout taken on baited hooks and returned to the water show 14 times the death rate of those taken on flies. Anglers wishing to conserve stocks or in search only of trophy fish are best advised to use flies. Hatcheries stock various waters with this trout, sometimes dropping them into lakes from planes. Restocking in the neighbourhood of the NCR on the Québec side in the 1930s suffered from poaching in quantity of these fish (McCuaig, 1935). The ready availability of this salmonid has made it a useful experimental fish for various physiological, biochemical, toxicological and other studies. Some reared stocks however show deformed or lost fins and distorted mouths. The possibility of "sea-ranching" Brook Trout in Atlantic Canada has been explored by releasing stream trout in estuarine areas to improve angling. In freshwater their preference for cool and clear water makes them susceptible to loss if waters are dammed, channelised and polluted or if banks are eroded deforested and overgrazed.
Lake Trout / Touladi
Salvelinus namaycush (Walbaum, 1792)
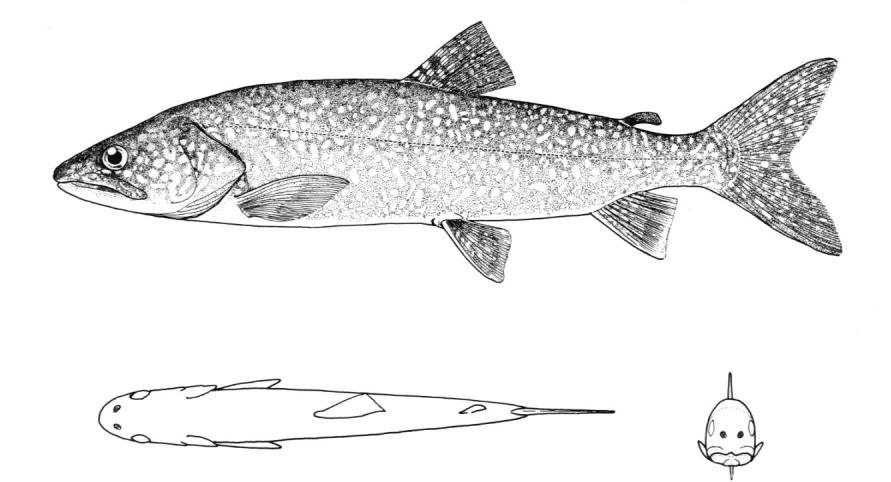


Taxonomy
Other common names include Lake Charr; Great Lakes, Forktail, Mackinaw, Salmon, Fat Lake, Grey or Mountain Trout; Laker, Landlocked Salmon, Siscowet, Taque, Togue, Truite de lac, Truite grise, Omble gris, Namaycush, Isok, Ihok, Nauktoq, Näluarryuk, Isuuq, Isuuraaryok, Isuuqiq, Siuktuuk, Sigguayaq, Ilortoq, Ivitaruk, Iqluq, and others. A Lake Trout and Brook Trout artificial hybrid is known as Splake, and is often stocked in lakes.
Key Characters
This species is distinguished by having 116-138 lateral line scales, 8-10 principal anal fin rays, teeth on the anterior end only of the vomer bone in the roof of the mouth, pectoral, pelvic and anal fins with a white leading edge but no black contrasting bar behind, head, flank, dorsal, adipose and caudal fin spots white to cream but never red, and the caudal fin has a deep fork.
Description
Dorsal fin principal rays 8-10, pectoral rays 12-17 and pelvic rays 8-11. Pyloric caeca 81-210 and gill rakers 16-26. Males and females have tiny nuptial tubercles around the anus.
Colour
Overall colour is light to dark green, dark olive, brown, grey or black with some fish very silvery masking spots. The back may have pale grey worm-tracks or vermiculations. The belly is dirty white to yellowish. Pectoral, pelvic and anal fins orange. Breeding males develop a black, flank stripe and more reddish brown body and reddish lower fins. The jaw tips and roof of the mouth become whitish. Young have 5-12 parr marks with the spaces between equal or greater in width. Fins are clear except dark bars develop on the dorsal fin of larger parr. Faber (1985a) illustrates a larva.
Size
Reaches 183.0 cm and 46.37 kg, possibly over 60.0 kg. The world, all-tackle angling record was caught in Great Bear Lake on 8 August 1970 by Larry Daunis and weighed 29.48 kg. The Ontario record as of the year 2000 weighed 28.6 kg. Small (1883) reports fish of close to 30 lbs (13.6 kg) from the NCR near Buckingham and a 24 inch fish is recorded from Lac Paquin (Ottawa Sun, 10 June2004).
Found from Labrador and Nova Scotia west to British Columbia and Alaska (but absent from most of the Hudson Bay lowlands, southern Saskatchewan, southeastern Alberta and southwestern B.C.), north to Banks, Victoria and Baffin islands (but rare on the last) and south to Idaho and Pennsylvania. Also introduced in the U.S.A., Europe, South America and New Zealand. It was introduced to Mississippi Lake in 1886 (Brown, 1984). Walker (1905) records the introduction of 30,000 young salmon trout, presumably this species, in "Meaches lake" (Meech or Meach Lake). Rubec (1975) records this species from lakes Lapêche, Philippe, Mousseau and Meach in the late 1800s, surviving in only Meach Lake.
Origin
Dumont (1982) considers populations in lakes of southern Québec to be relicts of anadromous populations living in the Champlain Sea, an arm of the Atlantic Ocean 11,900 years ago. These populations would have dispersed during the first phases of the marine invasion that followed the retreat of the glaciers. McAllister and Coad (1975) consider that this species survived glaciation in either an Atlantic or Mississippian refugium or perhaps both, as do Mandrak and Crossman (1992).
Habitat
Lake Trout are found, naturally enough, in lakes where they are solitary but may also be found in some northern rivers and rarely in brackish water. They prefer larger lakes, >100 ha, deeper than 12-14 m with pH>5.5. Southern populations are found only in deep lakes where they can retreat to cooler depths in summer since they prefer waters about 10°C, range 8-15°C. Distribution of this species is restricted by changes in the environment and interspecific competition, coupled with pollution and introduction of competitors (Dumont, 1982).
Age and Growth
Life span may exceed 53 years with maturity attained at 4-7 years in the south and 8-22 years in northern areas like Great Bear and Great Slave lakes. Growth varies over the wide range of this species, generally being slower in the north. Dymond (1939) notes that this species grow to a larger size when Cisco are present as food.
Food
Food includes plankton, sponges, aquatic insects, terrestrial insects when abundant, crustaceans, fishes including their own species and occasionally small mammals such as mice and shrews, and confused yellow warblers which fell onto the water surface in fog. The diet emphasis varies with habitat and plankton feeders grow more slowly than those which eat mostly fish. In smaller lakes fish may not be readily available in the cooler depths and such plankton as opossum shrimps are the mainstay. Opossum shrimps are 82-95% of the food volume of young (up to age 2) trout in Lake Superior but trout over 40 cm long have a diet which is 94% fish. Ciscoes are the most important fish in Lake Trout diet but sculpins are particularly important to younger trout. A wide variety of other fishes are taken as opportunity permits. Alevins in Heney Lake, north of the NCR, feed on the crustaceans Mysis relicta and Pontoporeia affinis (van Vliet and Qadri, 1970).
Reproduction
Spawning occurs from August to December, principally in October and early November in the Outaouais (Chabot, 1981d), and earlier in the north than the south. Temperatures are about 8-11°C (or 8.9-13.9°C after Thellen (1994), also for the Outaouais) and spawning occurs between 1900 and 2200 hours in the dark. Spawning is triggered, at least in part, by sudden temperature drops, cloud cover and onshore winds. Spawning lasts about 2-38 days at any one site, 7-38, mean 16, days in the Outaouais (Chabot, 1981d). Up to 18,051, 6.0 mm diameter eggs are shed over boulders, rubble, or clam shell beds, falling into crevices in moderately deep (to 60 m) to very shallow (a few centimetres) water. In the Outaouais, spawning occurs in depths less than 1 m and fish have been observed spawning with their backs out of the water (Chabot, 1981d). There is no redd construction unlike other salmons, trouts and chars. The spawning ground is cleaned with body or tail brushes or by rubbing with the snout. Most fish spawn in lakes but rarely river spawning is reported. The female spawns with 1-2 males or a group of males and females spawn together. The male nudges or nips the female, presses against her flank with the vents close together, the male erects his dorsal fin and they gape and quiver. Some populations home to spawning sites and disperse over 160 km, to return again in subsequent years. Spawning intervals for females vary, every third year in Great Bear Lake and every second year in Great Slave Lake. Eggs hatch in February to June depending on latitude and larvae are 15.0-16.0 mm long. Alevins have been noted in Lac David, Gatineau on 4 February (Chabot, 1981d). They leave the gravel at 20.0-25.0 mm as juveniles and swim near the lake bottom.
Importance
Sea Lampreys have caused a major decline in trout of the upper Great Lakes, having gained access via canals. DDT was another major factor in decline of this species, causing embryo death. Lake Trout also accumulate PCBs and other toxic chemicals and in 1976 Wisconsin banned human consumption of this fish from Lake Michigan. Overfishing has also caused a decline and natural populations of Lake Trout are extinct in lakes Ontario, Erie and Michigan and only 2 small, remnant stocks survive in Lake Huron. Lake Trout are now a small part of the Great Lakes sport fishery, having been replaced by introduced Rainbow Trout and Pacific salmons. Stocking of Lake Trout and lamprey control by chemicals have enabled Lake Superior trout to recover to some degree. Lake Trout are an important sport fish taken with flies or lures in cool seasons or by deep trolling in summer. Whole or cut fish can be used as bait when bottom fishing. Northern trout remain in cool, near-surface waters even in summer and deep trolling is not required. They can then be caught by spinning or on bucktails and streamers. Trout are also caught through the ice. The Northwest Territories have several expensive, trophy, sport fisheries requiring float plane transport to reach. They must be carefully managed to avoid over-exploitation by rod-and-line fishing. Catch-and-release is favoured with catch limits and fishery zones. Stockings in some Ontario lakes in efforts to improve catches over the natural return have been shown to be of no help. Limitations on take and shortened fishing seasons are probably the best way to maintain a sport fishery. There is a major planting programme in the Great Lakes with about 9 million yearlings being released annually. The flesh is white, pink or orange-red and is very tasty. Commercial catches in some lakes are taken with gillnets and are particularly important in the Northwest Territories. Lake Trout and Lake Whitefish make up 95% of the total catch there. The total Canadian catch in 1988 was 1050 tonnes.
Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates (and may include Brook Trout). For example, in 1881 the catch was 154,000 lbs (69,916 kg) in the Upper Ottawa and Gatineau lakes division, the highest recorded, in 1896 the catch from the Gatineau lakes was 98,100 lbs (44,537 kg), while in the Ottawa River from Carillon to Pontiac in Québec in 1898 the catch was 650 lbs (295 kg).
A Lake Trout fossil of Quaternary age (ca. 10,000 years ago) assigned to this species has been found in a clay nodule from Besserer's Springs (near Green Creek at Hiawatha Park in the NCR) (Gruchy, 1968; McAllister et al., 1981; Harington, 1972; 1983; McAllister et al., 1987
Percopsidae - Trout-perches - Omiscos
The Trout-perch Family contains only 2 species in temperate North American freshwaters, 1 of which is found in Canada, the other, the Sand Roller, being restricted to the Columbia River drainage in the U.S.A.
The name derives from their anatomy, which contains characters of both the trout or salmon-like fishes and the perch-like fishes. They include an adipose fin, weakly ctenoid scales, a scaleless head, weak spines at the origins of the dorsal, anal and pelvic fins, and 7-8 pelvic soft rays. Biology is summarised in the species account.
Trout-perch / Omisco
Percopsis omiscomaycus (Walbaum, 1792)
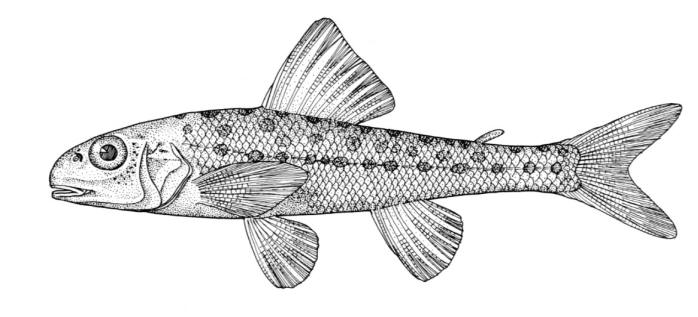
Taxonomy
Other common names include Silver Chub and Sand Roller.
Key Characters
The combination of weakly ctenoid scales, an adipose fin, weak spines in the dorsal, anal and pelvic fins, and the pelvic fin base under the pectoral fin is unique.
Description
Pyloric caeca number 7-14, arranged in 2 rows on each side of the intestine. Dorsal fin with 2 weak spines, 9-12 soft rays. Anal fin with 1 weak spine and 5-8 soft rays. Pelvic fin with 1 weak spine and 7-9 soft rays. Pectoral fin rays 12-15. Scales 41-60 in a complete lateral line. Gill rakers are short with small teeth and number 9-12.
Colour
Background colour is silvery with 5 rows of black spots. There are 9-12 spots on the back mid-line, 7-12 weak spots on the upper flank and 8-13 obvious spots or blotches along the mid-flank. The back may have a purplish tinge or be yellowish, brownish or greyish. Fins are mostly clear with some faint pigment along fin rays. Large cavities on the cheek and lower jaw are silvery-white. The body is often translucent and the silvery-white internal body cavity lining can be seen through the skin.
Size
Reaches 20 cm.
Found in central and northern North America. In Canada it occurs from western Québec, including around James Bay, throughout Ontario, Manitoba and Alberta, but only in northeastern British Columbia, and in the eastern Yukon, Nunavut and the Northwest Territories.
Origin
This species entered the NCR from a Mississippian refugium (Dadswell, 1972) or possibly a Beringian refugium (Mandrak and Crossman, 1992).
Habitat
Trout-perch favour deeper water of lakes (down to 60 m) but enter streams to spawn in the east. In the NCR it has been caught in streams with slow to medium currents, mud, debris or rock bottoms and light brown water. Their preferred temperature is 15-16°C and they are tolerant of turbidity.
Age and Growth
Males live to 3, and occasionally to 4, years in Lake Champlain with females living to 6 years. Maximum age elsewhere is reported as 8 years but maturity can be reached as early as 1 year. Females are larger than males.
Food
Food includes aquatic insects, crustaceans and small fish such as darters and minnows, perhaps taken mostly at night during an inshore migration. Trout-perch are an important food for Northern Pike, Walleye, Burbot, Lake Trout, Brook Trout, Sauger, Yellow Perch and Freshwater Drum. Often the only evidence of Trout-perch in predator stomachs is the pyloric caeca, which produce, and are therefore resistant to, digestive enzymes.
Reproduction
Comtois et al. (2006) report two females in reproductive stage V (spawning) in the lower Gatineau River on the 6 May at 10ºC. Spawning occurs in streams or over sand and gravel in lake shallows. Most southern Canadian populations are believed to migrate to spawn in streams in May and then return to lakes. Lake spawning may run from May to August. In the north, ripe males and females have been caught in June and July. Egg diameters are up to 1.85 mm and egg numbers to 1825. Eggs are heavier than water and stick to the bottom.
Importance
Occasionally used as bait but otherwise not of direct, commercial importance. One important function of this species may be as a nutrient transporter. Lake Trout are confined to cool depths of lakes and cannot feed in the food-rich but warm shallows. However Trout-perch feed in the shallows at night and return to deep water for the day where they fall prey to the Lake Trout.
Cods are mostly northern marine fishes with 1 species in freshwater of North American and Eurasia and a few southern hemisphere species. There are about 30 species and in Canada 24 species with 1 in the NCR.
Cods have a first dorsal fin behind the head and 1-3 distinct dorsal fins. There are 1-2 anal fins. There are no fin spines. The caudal fin usually extends around the dorsal and ventral tip of the caudal peduncle often framing a pointed end to the body. The vomer bone in the roof of the mouth bears teeth. The swim bladder is not connected to the auditory capsules and has 2 slender, anterior processes. There is usually a barbel at the tip of the lower jaw. Scales are small and cycloid. There is an obvious lateral line.
Most cods live on or near the bottom in cold shelf and slope waters of the sea in large schools and are of immense commercial importance as food and sport fishes. Eggs and larvae are usually pelagic. Egg production can exceed 60 million in some species. Adults feed on other fishes and various invertebrates. Cods are the principal food fish consumed by humans.
A fossil of a freshwater to marine species, Microgadus tomcod (Walbaum, 1792) has been found in peri-Champlain Sea nodules at Hiawatha Park on the Ottawa River and from Covent Glen, Ottawa and and the mostly marine species Gadus morhua Linnaeus, 1758 has been found at Eardley (McAllister et al., 1981; 1987).
Burbot / Lotte
Lota lota (Linnaeus, 1758)
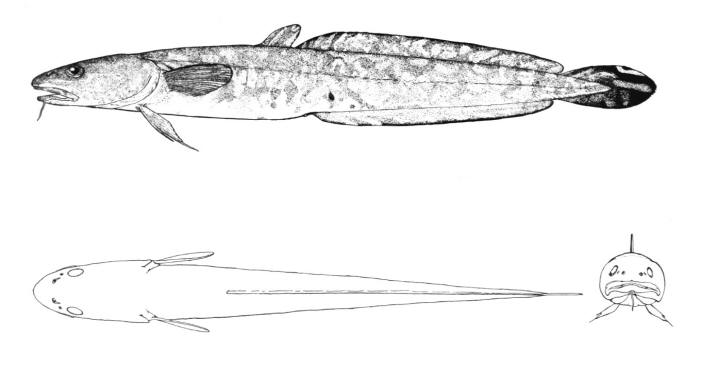


Taxonomy
Other common names include Ling, Sand Ling, Eelpout, American Burbot, Freshwater Cod, Mother-of-Eels, Gudgeon, Maria, Methy, Freshwater Cusk, Spineless Cat, Dogfish, Lawyer, Lush, Loche, Queue d'anguille, Freshwater Eel, Lotte de rivière, Titaliq, Nätarrnaq, Tiktabek, Shulukpaoluk, Nettârnak. The name lawyer is said to be in allusion to its slipperiness or to its aggressive behaviour and voracious feeding. The word "burbot" is derived from the Middle French "bourbotte" from the verb bourbeter, to wallow in mud.
Key Characters
This is the only freshwater member of the Cod Family and its shape is distinctive. It has 2 dorsal fins, 1 anal fin, pelvic fins anterior to the pectorals and a single barbel at the chin tip.
Description
There are large, tubular nostrils. The dorsal and anal fins are fleshy and rays cannot be counted without dissection. First dorsal fin rays 7-16, second dorsal rays 60-94. Anal fin rays 52-86, pectoral rays 15-24 and pelvic rays 5-9. The embedded scales are very small. Pyloric caeca 31-168.
Colour
Overall colour varies from yellow-brown to brown or dark olive with black mottling and blotching. The belly is yellowish-white. Some fish may be uniform brown, purplish-black or black. The pelvic fins are pale and other fins are dark and mottled. The second dorsal, caudal and anal fins have a dark, submarginal band while the margin is bright yellow or orange. Young fish are usually dark and can have a white anal fin with a black edge and black rays in the pectoral fin. Faber (1985a) illustrates the larva.
Size
Reaches 1.52 m and 34.0 kg, possibly to 2.1 m and 36.0 kg. The world, all-tackle, angling record weighed 11.23 kg and came from Lake Louise, Alaska in 1976. A 10.2 kg fish was caught in Little Athapapuskow Lake, Manitoba.
Found throughout Canada except coastal British Columbia, extreme northeastern N.W.T., the Arctic Islands, the outer coast of Québec and Labrador, Nova Scotia, P.E.I. and Newfoundland. Also in the northern U.S.A. and across all of northern Europe.
Origin
This species entered the NCR from a Mississippian or possibly an Atlantic coastal refugium (McAllister and Coad, 1975) or possibly a Beringian refugium (Mandrak and Crossman, 1992).
Habitat
Burbot are found in lakes down to 214 m and in large, cool rivers, generally preferring water colder than 18°C (other reports give a preferred temperature of 13°C or below and even 21.2°C). A preferred habitat is under rocks, among roots or in holes in banks. In large lakes, trenches are excavated for a habitat. In rivers turbid water is preferred. Generally they are sedentary. They may move into shallow water at night in summer and there may be long-distance spawning migrations. Young Burbot can be common in lake shallows and streams. In the NCR, they are most commonly caught in rivers and streams. In the Mississippi River, one specimen was caught among rocks in turbulent water flow.
Age and Growth
Females are larger than males and often mature later. Maturity is usually attained at 3-4 years and life span is up to 20 years. Not all adults spawn each year. In Lake Simcoe, Ontario males mature at 34.3 cm and 255 g and females at 41.9 cm and 680 g, both at age 3.
Food
Food, taken at night, is aquatic insects, crayfish, molluscs and other invertebrates when young (up to 50 cm) but older burbot eat mostly fishes with some opossum shrimps and other crustaceans, the latter especially in winter. Young-of-the-year Burbot in the Ottawa River at Kettle Island and Upper Duck River fed predominately on amphipods and darters but also took isopods, worms, snails, fly larvae and dragonfly larvae (Hanson and Qadri, 1980b). The Burbot is a competitor with many fishes for food, especially since it feeds indiscriminately and voraciously on whatever is available. The barbel and the pelvic fins are used to taste food before ingestion, even ejecting a food item from the mouth and passing it back to the pelvics several times before finally consuming it. Young Burbot are eaten by various other fishes.
Reproduction
Spawning takes place from January to March under ice during a short 2-3 week spawning season. There is a movement into shallow water for spawning, usually at depths less than 3 m, over sand, gravel or cobbles near the shore or on shoals in lakes, and in main channel rivers and streams where velocity is low. Males arrive first on the spawning grounds followed in 3-4 days by females. Water temperatures are 0.6-1.7°C. Up to 12 fish form a moving, wriggling ball over the bottom at night. Eggs are shed into the water column above the substrate, fertilised and left unattended. Eggs are up to 1.9 mm in diameter and number as many as 1,362,077, perhaps up to 3.5 million. They are semipelagic but gradually sink until lodging in gaps between sand and gravel. They take up to 18 weeks to hatch at temperatures below 2°C. Larvae are 3.0-4.0 mm long and live in open water.
Importance
Burbot have been used for fish meal, oil and food for animals raised for fur. It has white, flaky flesh and is good eating but has not found general acceptance as a food fish. It is said to taste like "barbotte" (Ameirus nebulosus), a more familiar food species in the NCR. Smoked Burbot livers are a delicacy in Europe. Anglers often catch Burbot when ice fishing for Lake Trout or other species. Sarsfield (1975a) records a 5½ lb "ling" caught in the Annual Ice Fishing Derby at Britannia Park. Burbot can be a nuisance to commercial fisheries, eating other commercial fishes caught in gillnets or clogging gillnets and wasting time in removing them. A report in 1903 attributed the loss of pike, bass and perch from night lines set in the Mississippi River of the NCR to "ling", only the heads of the fishes remaining on the hooks (Brown, 1984). It is reputed to eat the eggs of more commercially valuable fishes (Prince and Halkett, 1906).
Atherinopsidae - New World Silversides - Poissons d' argent
Silversides are found mostly in temperate to tropical seas with some species in freshwaters. There are about 165 species with 3 in Canada and 1 in the NCR. The NCR spccies was formerly placed in the family Atherinidae.
These small, elongate, silvery fishes have a short, spiny first dorsal fin widely separated from the second dorsal fin. The second dorsal often has a spine in front of the soft rays. Pelvic fins are usually abdominal and have 1 spine and 5 soft rays. Pectoral fins are high on the flank. The anal fin has 1-3 spines preceding the soft rays. Cycloid scales are usually large, extend onto the head and there is no lateral line although there may be a row of pits, 1 to each scale. Many species have a broad, silvery, iridescent lateral stripe which turns black in preserved fish. The oblique, terminal mouth is small as are the teeth. The swimbladder is not connected to the gut.
These fishes are found in large schools but are not commercially important in a direct sense. However they are major bait and forage fish for commercial species. They are usually found in shallow, inshore waters. Eggs of many species have filamentous outgrowths for attachment to seaweed, sand or rocks. Food is principally plankton. Some species from tropical waters are sold as aquarium fishes.
Brook Silverside / Crayon d'argent
Labidesthes sicculus (Cope, 1865)
Taxonomy
Other common names include Skipjack, Topwater, Friar and Glassfish.
Key Characters
This is the only freshwater family member in Canada and is easily recognised by the short, spiny, centrally placed first dorsal fin and longer soft second dorsal fin, both over a long anal fin.
Description
First dorsal fin spines 3-6 (this fin is small and easily missed), second dorsal fin with 1 spine and 9-13 soft rays. Anal fin with 1 spine and 20-27 soft rays and pectoral rays 12-13. There are 74-99 scales in lateral series, only a few of them pored. There are 24-29 long gill rakers.
Colour
Overall translucent with some internal organs visible, particularly in small fish. Preserved fish are opaque. The back is pale green to olive or yellowish, the flank has an iridescent silvery stripe outlined by a black stripe above. The belly is white or silvery. Scales on the back are outlined with dark spots. Fins are translucent but breeding males have a black tip to the first dorsal fin. Faber (1984c) illustrates a larva.
Size
Attains 11.2 cm.
Found from the westernmost Québec in the Ottawa River and in the Rideau River both in the NCR, and the upper St. Lawrence River across southern Ontario to Georgian Bay and Lake Huron west to Minnesota, south to Gulf of Mexico drainages and Florida.
Origin
This species entered the NCR from a Mississippian refugium via a postglacial dispersal route.
Habitat
This silverside is found in surface waters in large schools in lakes and large rivers, but not brooks. The schools are not maintained through the night. Young fish avoid any solid objects perhaps as a mechanism to keep them out of shallow water where potential predators are found. The young swim with their heads touching the surface film of the water. Larger fish enter shallows. They are particularly intolerant of turbidity, a condition enhanced by urban development and agricultural activities. Their preferred temperature is 24.5°C.
Age and Growth
Life span seldom reaches 2 years and most fish die after spawning a year after being born. Growth is extremely rapid, 0.4 mm per day, reaching adult size in only 3 months. A one-year life cycle leaves this species susceptible to local extinction should some catastrophic event occur. Such an event would include ice build-up in shallow water.
Food
Food is water fleas, midge larvae, other insects and plankton, and flying insects and spiders which land on the water. These items are taken with a snap and some flying insects are taken by a leap out of the water. Leaps may be up to 10 times body length. Silversides are food for many sport and other fishes.
Reproduction
Spawning occurs in spring and summer (May-August) at 17-23°C over vegetation or gravel. Spawning is protracted, lasting at least 41 days. Males may defend a territory against other males. Territories eventually break down. Females are pursued by 1 or more males and may leap from the water followed by the males to a height of 2-3 cm landing up to 10 cm away. When caught by a male, the 2 fish glide downward with their bellies in contact and eggs are thought to be extruded and fertilised. Orange eggs are up to 1.4 mm in diameter and have 1-3 greatly elongate, adhesive filaments to attach them to vegetation. Some are deposited on floats and anchor ropes. There are up to 785 mature eggs present in a female at any time. Silversides in Florida have internal fertilisation and sperm are transferred by a short, genital palp when ventral surfaces of a breeding pair are pressed together. Whether this occurs in northern populations is unknown. Larvae hatch at 4.0-5.0 mm.
Importance
This species has been used as bait by anglers although it is not a legal bait fish in Ontario (Goodchild, 1990b). This species was placed in the "Not at Risk" category in 1989 by the Committee on the Status of Endangered Wildlife in Canada.
Fundulidae - Topminnows - Fondules
Topminnows or Killifishes are found in fresh, brackish and coastal marine waters of North and Central America where there are about 48 species. There are 3 species in Canada, 1 on the Atlantic coast and in Atlantic drainages, 1 in Arctic and Atlantic drainages including brackish waters of the Atlantic coast, and 1 in freshwaters of the Atlantic drainage.
These small fishes are distinguished by a series of osteological characters such as the autopalatine bone projecting anterior to the lateral ethmoid and the anteriorly directed ventral arms of the maxillary bones of the upper jaw often have strong hooks. Scales are cycloid and extend onto the top of the head. The snout is pointed and elongate compared to related families. The head is flattened on top and the mouth upturned and protrusible. Lateral lines are absent on the body but can be well-developed on the head. The caudal fin is truncate and fins lack spines. Pelvic fins are abdominal. The swimbladder lacks a duct to the gut.
"Killifish" is derived from a Dutch word for a creek or channel in reference to their habitat.
Topminnows lay eggs. Food is usually surface items taken by the oblique mouth. The flattened head may be an adaptation to poor oxygen conditions enabling topminnows to live as close to air-water interface as possible in oxygen-rich conditions. Some are colourful and their tolerance of warm temperatures and wide salinities make them attractive aquarium fishes. Others have been used extensively as experimental animals.
Banded Killifish / Fondule barré
Fundulus diaphanus (Le Sueur, 1817)
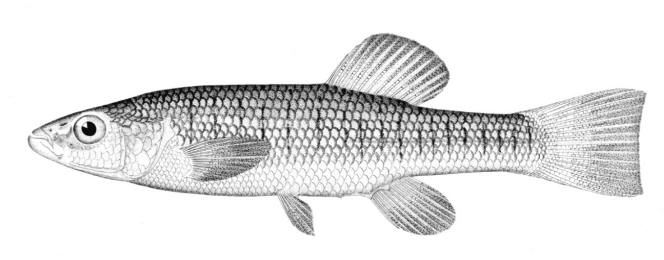
Taxonomy
Other common names include Freshwater Mummichog, Freshwater Killy, Grayback, Topminnow, Eastern and Western Banded Killifish, Menona Killifish, Barred Minnow, Hardhead and Petit barré. There are two subspecies, Fundulus diaphanus diaphanus found eastwards from eastern Lake Ontario and Fundulus diaphanus menona Jordan and Copeland in Jordan, 1877 found westwards from Lake Erie. These two subspecies intergrade in the upper St. Lawrence River and Lake Ontario. NCR fish have not been investigated to determine their subspecies.
Key Characters
This species is the only topminnow in the NCR and is distinguished by having an upturned mouth and scales on the head and cheeks.
Description
Dorsal fin rays 10-15, anal rays 9-13 and pectoral rays 14-19. Scales in lateral series 35-53, no lateral line pores. Gill rakers small, numbering 4-7. Males have larger dorsal and anal fins than females. The 2 subspecies are distinguished by the western subspecies having fewer lateral scales, fewer dorsal, anal and pectoral fin rays, and a stripe through the bars on the caudal peduncle.
Colour
Back brown to olive-green or olive-yellow, silvery on the flanks and white or yellow on the belly. There are 8-22 green-brown bars along the flank. Males have more and wider bars than females. Breeding males develop a green-gold dorsal fin with faint black bars, stronger, wide, green flank bars, a yellow throat and have an intense blue-green back. Faber (1984c) illustrates a larva.
Size
Attains 11.4 cm total length.
Found in Arctic and Atlantic drainages of Canada including brackish waters of the Atlantic coast. It is found from southwestern and southeastern Newfoundland, Anticosti Island, rarely on the north shore of the Gulf of St. Lawrence, and from the southern Gulf of St. Lawrence south to South Carolina. It extends up the St. Lawrence valley, across southern Ontario and in the southern Great Lakes basin but not north of Lake Superior, and is in Lake of the Woods and southern Manitoba and as far west as eastern Montana.
Origin
This species entered the NCR from possibly an Atlantic coastal refugium, a Mississippian or a Missourian refugium (Mandrak and Crossman, 1992). The presence of this species in Gatineau Park may be from discarded bait fish brought in by anglers (Rubec, 1975a).
Habitat
In fresh waters, Banded Killifish are found in schools in quieter parts of lakes and slow rivers over bedrock, boulders, sand, gravel, silt, mud or detritus near aquatic plants. Water clarity is important as this fish feeds visually. It tolerates low oxygen and high temperatures (above 38°C, although preferred temperature is 21.0°C in Nova Scotia and 28.6°C in Pennsylvania). When disturbed this fish will dive into the substrate at an angle of 45°, burying itself by vigorous lateral body movements. It may remain wholly or partially buried for less than a minute to more than 2 hours.
Age and Growth
Life span is up to 4 years with maturity as early as 1 year.
Food
Food is taken at all levels in the water column despite the dorsally positioned mouth and includes insect larvae, crustaceans, molluscs, flatworms, and flying insects at the surface. Feeding occurs mainly in the afternoon with a shorter period starting just before dawn. Surface feeding causes ripples. It is eaten by various fishes and birds.
Reproduction
Spawning occurs in spring and summer, with females in spawning condition noted in the Ottawa River in June when temperatures were around 23°C (McAllister and Coad, 1975). In Québec spawning occurs in mid-July to August when fish are 2 years old and water temperatures are 21-25°C. Elsewhere maturity at 1 year has been recorded. Males establish territories in weedy areas and fight off other males. The male pursues a female until she extrudes an egg which hangs from her body by a filament. The male redoubles his pursuit and drives the female into vegetation, presses against the female using his dorsal and anal fins, and the female extrudes 5-10 more eggs. The male quivers, bends his body and fertilises the eggs. The eggs separate from the main thread and each other and become tangled in weeds by their own threads. This mating takes only 15-30 seconds and is repeated several times over 5 minutes until about 50 eggs are deposited. Egg diameters are up to 2.1 mm and a female can contain 426 yellow-orange to orange eggs. Eggs hatch in 11-12 days at 22-27°C and larvae are 5.0-6.0 mm long.
Importance
The Banded Killifish has been used as bait in the Maritimes since it is easily transported, reputedly for days packed only in leaves or moss in a can. It is not classified as a bait fish in Ontario and so it is illegal to use it as one (Houston, 1990). This species was placed in the "Not at Risk" category in 1989 by the Committee on the Status of Endangered Wildlife in Canada.
Gasterosteidae - Sticklebacks - Épinoches
Sticklebacks are found in coastal marine and fresh waters of the Northern Hemisphere. The number of species in the family is more than 7 with 5 named from Canada (but see below) and 3 in the NCR. The record by Small (1883) of the Fourspine Stickleback, Apeltes quadracus (Mitchill, 1815), in the NCR is an error as this is an Atlantic coast species and is, rare in fresh waters.
The family is characterised by 3-16 isolated spines in front of the soft dorsal fin, which has 6-14 rays, bony plates often present along the flank, the pelvic fin has 1 spine and 0-3 soft rays, caudal rays usually 12, slender caudal peduncle, small mouth with teeth, a swimbladder not connected to the gut, 3-4 branchiostegal rays, and various other osteological characters.
The number of species is uncertain and may not be readily explicable using the conventional, scientific naming system. The Threespine Stickleback as currently defined is a species complex containing populations which act as good species. This would require a major revisionary study given the great diversity shown by populations around the Northern Hemisphere. There is variation in colour, body form, maximum age (8 years as opposed to a usual 2-4 years), spine numbers and development, and plate numbers. These variations in anatomy are matched by variations in biology such as habitat, feeding and reproduction. In British Columbia, a centre for unusual sticklebacks, there is the giant Mayer Lake form, the unarmoured Boulton Lake form, the black Drizzle Lake form (all on the Queen Charlotte Islands), the Enos stickleback, and the Heisholt or Texada stickleback of Paxton, Priest, Emily and Balkwill lakes on Texada Island. The White Stickleback is the only unusual Atlantic coast form to receive detailed attention.
This variation in behaviour, biology and in speciation has attracted extensive studies by scientists and makes these small fishes, which have no commercial value, particularly important. Some of the variation is owing to environmental factors while some has a genetic basis. Several books have been devoted to them and thousands of scientific studies. Sticklebacks make excellent aquarium fishes. Their reproductive behaviour is complex, involving courtship and nest building. Some populations are anadromous and enter fresh water to breed.
Brook Stickleback / Épinoche à cinq épines
Culaea inconstans (Kirtland, 1840)

Taxonomy
Other common names include Five-spined, Black, Variable, Common, or Six-spined Stickleback and Pinfish.
Key Characters
This species is distinguished by having 4-7 short dorsal fin spines, no obvious bony plates on the flank and all spines are smaller than the eye diameter.
Description
Soft dorsal fin rays 8-13, soft anal rays 7-12 after 1 spine, pectoral rays 9-12 and pelvic fin with 1 spine and 1 soft ray. There are 30-36 tiny plates along the mid-flank. Many populations in Alberta lack part or all of the pelvic girdle and fin, and various populations across Canada show the occasional fish with reduced pelvic skeletons although this has not been found in the NCR
Colour
The back and flanks are olive-green, the flanks with lighter spots or short wavy lines. The belly is whitish-yellow or silvery-white. The dorsal and anal fin membranes are dusky. Breeding males are jet black, sometimes tinged with copper, and the pelvic fins have a red tinge. Females are a light green but develop a dark and light pattern when spawning. Peritoneum silvery with many melanophores. Faber (1984c) illustrates a larva.
Size
Reaches 8.7 cm fork length.
Found from western Nova Scotia and New Brunswick west to northeastern British Columbia and the Mackenzie River basin. It is absent from northern Saskatchewan and Manitoba, Nunavut and the Yukon. In the U.S.A. south to Indiana and Nebraska and as a relict in New Mexico.
Origin
This species entered the NCR from a Mississippian refugium or possibly a Missourian refugium (Mandrak and Crossman, 1992).
Habitat
Brook Sticklebacks are found in small streams, bogs, ponds or lakes down to 55 m. The water is usually still or slow and bottoms are mud, detritus, sand gravel or rocks. The water can be clear or tea-coloured. They prefer heavy vegetation and are tolerant of low oxygen. Their preferred temperature is 21.3°C. These fish can spawn in temporary steams which can then dry up and strand them. Gas bubbles under ice in winter prolong survival of this fish since it can take advantage of a microlayer of water with higher oxygen next to the bubble. However in Kemptville Creek, large schools move upstream in spring to populate areas abandoned in winter on account of low oxygen levels (Schueler et al., 1992). Brook Sticklebacks may burrow into silt, remaining covered for more than half an hour. In some instances this behaviour is a search for food.
Age and Growth
Life span is 3 years at most and fish mature at 1 year. Most populations are annual fish which die after spawning in their second summer.
Food
Food is aquatic insects, crustaceans, snails, worms, algae, sponges and fish eggs and fry including those of Brook Sticklebacks. In Manitoba vegetated stream margins most feeding occurs between noon and 8:00 p.m. Feeding consists of five phases - swim, hover, aim, dart and handle. The hover or aim phases involve deciding whether to eat or reject the item seized. "Food fighting" occurs, especially under crowded conditions. Large food items are shaken apart and this causes up to 7 sticklebacks to compete for large food fragments. This process establishes a hierarchy among the fish. These sticklebacks are eaten by a variety of other fishes, leeches, birds, shrews, muskrats, and large larvae of water beetles and dragonflies. Pelvic spines protect against fish predators by increasing the size of mouth which can handle them. However water beetles seem to favour eating those sticklebacks with pelvic spines and are less successful at grasping spineless fish. This may be due to the closer approaches to the predator made by spiny fish; those lacking spines are more wary. Fish without spines compensate by a changed behaviour, perhaps taking more advantage of vegetation as shelter.
Reproduction
Spawning takes place from April to August at 4.5-21°C, usually at 8°C or warmer. Spawning is later in the north than the south. Males arrive on the spawning ground before females and build and defend nests. Ritualised displays against other males involve swimming parallel head to head or head to tail, fluttering their bodies and with spines erected. This is often followed by a quick attack. Males darken during this display and attack and develop black bands through their eyes. The nest is built on stems of vegetation near, or more rarely on, the bottom using dead and living fragments of vegetation, glued together with the white kidney secretions. The nest is a round barrel, up to 5.0 cm in diameter with a single opening. The male courts a female with nips, nudges and butts. Once she enters the nest he stimulates egg laying by prodding her belly and caudal peduncle. Eggs are yellow, adhesive and about 1.3 mm in diameter. Females produce on average up to 1926 eggs in a season in Manitoba, spawning 214 eggs every 3 days for 28 days. The female leaves the nest by pushing out, creating an exit hole which the male tries to repair. The female is driven away by the male. As more females are induced to spawn he enlarges his nest. The male looks after the eggs by fanning them with his pectoral fins. The young are also defended until they leave the nest. Larvae are 5.0-6.0 mm long at hatching.
Importance
This species has occasionally been used for bait in Québec.
Threespine Stickleback / Épinoche à trois épines
Gasterosteus aculeatus Linnaeus, 1758
Taxonomy
Other common names include Twospine, Common, Eastern, European or New York Stickleback; Banstickle, Panstickle, Pinfish, Tiddler, Kakilusuk, Kakilaychok, Katilautik and Kakilishek.
Key Characters
This species is distinguished by having usually 3 (range normally 2-4) dorsal fin spines, usually well-developed flank plates, 1 pelvic spine and 1 soft ray with a single cusp at the base, and colour.
Description
Second dorsal fin soft 7-14, anal fin with 1 spine and 6-11 soft rays, and 8-11 pectoral rays. Gill rakers number 23-25. Flank plates may be restricted to the anterior body under the first 2 dorsal spines or continuous along the whole flank forming a keel on the caudal peduncle. Most marine populations have complete series of plates (up to 37) while freshwater populations may have a complete series, only a few anterior plates, or both these extremes and intermediates. NCR fishes have a complete row of plates (although not as heavy as in marine fish) or there may be some absent before the caudal peduncle keel (the partial morph), and rarely are fish found with no keel and only anterior plates (Coad, 1985b). A population in a stream near Eardley was composed wholly of the partial morph, an unusual finding as the rare partial morph populations are usually found in lakes less than 10 km from the sea. Some fishes in the NCR show fusions of vertebrae, as revealed by x-rays (Coad, 1974). This anomaly may be due to adverse temperatures during development.
Colour
Marine populations are more silvery on the flanks than freshwater ones which are more olive. Generally the back is green-brown, olive or grey to blue-black, flanks olive to silvery and the belly silvery-white. Fins are generally clear. Breeding males develop a red belly and throat, blue sides and have bright blue eyes. Some populations in the Queen Charlotte Islands have lost or reduced red throat and belly colour which may be related to diets deficient in carotenoids.
Size
Attains 10.2 cm.
Found on the Hudson Bay and southern Baffin Island coasts and nearby freshwaters and south on the Atlantic coast and nearby freshwaters to Chesapeake Bay. Also inland to the Ottawa River and Lake Ontario. Introduced to Hasse Lake, Alberta and to lakes Huron and Michigan and possibly Lake Superior; also introduced as bait fish to various localities in southern Ontario. On the Pacific coast and nearby freshwaters from the Bering Strait of Alaska to California including British Columbia. Also in Europe and western Asia from the Arctic south to Syria and in the western Pacific Ocean south to Korea. In the NCR, this species is more commonly encountered in Québec waters as, despite extensive and intensive sampling, there are few NCR Ontario records although these are confirmed by specimens in the Canadian Museum of Nature collections.
Origin
This species is a relict of the Champlain Sea episode, when the sea invaded the Ottawa Valley about 11,800 B.P. (Dymond, 1939; Dadswell in Coad and McAllister, 1975), having survived in an Atlantic coastal refugium. A fossil stickleback from Green's Creek was dated to about 10,000 years ago (McAllister et al., 1981; 1987).
Habitat
These sticklebacks inhabit lakes, ponds, rivers and streams. Marine and lake fish can be pelagic. Their preferred temperature is 9-12°C.
Age and Growth
Life span is a little over 3 years although some fish probably live only 1 year and a few months, dying after they spawn.
Food
Food is various crustaceans, aquatic and terrestrial insects, snails, worms, fish eggs and fry including their own species, and a wide variety of other available organisms taken both on the bottom or pelagically. Females feed mostly in the early morning at a marine site at Isle Verte, Québec. Females are important cannibals, forming raiding schools of up to 300 fish which overwhelm the nest-defending male. A male will divert the raiders by diving to the bottom and rooting in the mud away from his nest, as though feeding on the nest of another stickleback, by snout tapping on the bottom as though showing a nest to a female, or by swimming away high above the bottom on his side, silver flanks shimmering in the light, which leads the females away from his nest. A male may even pick up a dead stickleback or a discarded cigarette butt, bright underwater objects, and swim erratically away chased by the other sticklebacks which try to steal this attractive object. Some females known as courtship cannibals, not yet ready to spawn, mimic reproductive females and court males to gain access to eggs already in the nest on which they feed. Many fishes and birds, and even snakes, seals and small mammals, feed on sticklebacks despite their protective spines which are locked erect when they are disturbed. Spines and body plates are concentrated at the anterior end of the body and at the centre of mass. This is where most predators strike and even tail caught fish are manipulated so as to be swallowed head first incurring most injuries anteriorly. Loss of posterior plates may increase swimming ability and enable sticklebacks to escape predators where shelter is available or predators have similar swimming speed. Open waters and fast predators would encourage more plates.
Reproduction
Spawning occurs from April to October, varying with locality over the wide range of this species. Males in full nuptial colouration defending a nest area have been observed on rocky shores of Pink Lake on 18 May. NCR fish taken in June had eggs 1.2 mm in diameter indicating spawning in June-July (McAllister and Coad, 1975). The male parental cycle at Isle Verte lasts 9-15 days with female interspawning intervals of 19 days. Males and females only complete one spawning here though laboratory studies show males capable of 5 reproductive cycles and females of producing a clutch of eggs every 3-4 days. Harsh physical conditions are probably the cause. The male builds a barrel-shaped nest in shallow, sandy areas from plant fragments glued together on the bottom. The nest is in an open area but near vegetation. The nest has an opening at each end. The male has a complex courtship dance with zig-zag motions and a leading motion to the nest. A responsive female adopts a submissive head up position, which also reveals the egg-swollen belly. The male pokes his snout at the nest to indicate its position to the female, tipping his head sideways to display the bright red throat. The male jabs the female with his snout through the nest wall after she enters to stimulate egg release. He then follows the female through the nest to fertilise the eggs and drives the female away. Some males steal eggs from a rival male and some eggs in a nest are fertilised by a "sneaker" male. The parenthood of eggs in nests, which determines these observations, was confirmed by DNA "fingerprinting". In Crystal Lake, B.C. a female initiates courtship by "jumping" on the back of a male in open water, squirming and pressing against his erect dorsal spines. This courtship is not as conspicuous as zig-zag dancing and serves to avoid egg raids by large, bottom-feeding female schools. The male can search the surrounding water for raiders before leading the female down to his nest. Several females may spawn in one nest which can contain up to 1026, yellowish 1.8 mm diameter eggs. Some females at Isle Verte have up to 838 eggs on average. The male guards and fans the eggs and guards the fry. Females ofter cannibalise eggs and the stress of defending against female attacks shortens male life span. Many males do not construct nests and many which do are unable to attract females. Nest cover and aggression are probably important factors in male success.
Importance
This species occasionally appears in local newspaper reports and appears on information signboards at Pink Lake, Gatineau Park (Coad, 1985b). It is of considerable importance to scientists despite its small size and lack of direct economic importance. Threespine Sticklebacks have been studied extensively for the light they throw on speciation and evolution, and on fish behaviour. It helps that they are easily maintained and bred in aquaria.
One aspect of stickleback variation is the number of plates along the flank. The classical situation has been examined in the Little Campbell River, B.C. A fully-plated marine form enters freshwater to spawn in the river. There it encounters a low-plated freshwater form and hybrids with an intermediate plate count result in a narrow contact zone. Freshwater males zig-zag more, glue nests more and bite less than marine males. Reproductive behaviour also differs. The 2 forms act as good species. However low and fully-plated forms co-exist in lakes and appear to be a single species. A population near Eardley, Québec in the NCR is composed almost entirely of partially-plated fish, a rare occurrence, only the fourth in eastern North America where such populations are usually in lakes 10 km or less from the sea (Coad, 1983; 1985b). In the absence of parental forms (fully- and low-plated), the occurrence of a population comprised only of partially-plated fish shows that the classical situation does not always apply (Coad, 1985b). Most other NCR populations are comprised of fully-plated or a mix of fully- and partially-plated fish (sample sizes are small for some populations, making population description uncertain). In the NCR, it is surmised that the fully-plated marine populations, relicts of the Champlain Sea, have variously retained all or lost some of their plates - but the reasons are unknown. Perhaps environmental factors play a part.
The species is often referred to as the "Gasterosteus aculeatus complex" because the fishes included under the scientific name include some forms which act as good species but have not yet been adequately defined. Some of these unusual forms, believed to be good species are described as the Enos, giant and Texada sticklebacks. There is also a stickleback in Nova Scotia marine waters which has unique male breeding colours, the white stickleback. Other Pacific coast populations variously have reduced or absent pelvic skeletons, plates, and dorsal fin spines, black rather than red breeding colours or are unusually large. Benthic forms have large, deep bodies, wide mouths and few, short gill rakers while limnetic forms have the opposite characters - both forms are found in Paxton Lake on Texada Island, B.C. Other characters, such as vertebral counts, show as much variation between localities from one river system in the Queen Charlotte Islands as in all of Europe. Rapid speciation rates are common in these sticklebacks since the age of the lakes in which they live is about 9500 years. Local selection, related to predators in a fish poor environment and feeding environments, has resulted in a wide range of characters with a genetic basis. Unarmoured populations of the Threespine Stickleback, which may be a distinct species, are reported from lakes on Graham Island in the Queen Charlotte Islands, B.C. They were given "rare" status in 1983 by the Committee on the Status of Endangered Wildlife in Canada. All the unusual populations of threespines in British Columbia should be protected as simple alterations of habitat or introduction of another fish species may upset the delicate balance which has led to their evolution.
Fossils of Quaternary age (ca. 10,000 years ago) have been found in clay nodules from Green Creek in the NCR, relicts of the Champlain Sea (McAllister et al., 1981; Harington, 1972; 1983; McAllister et al., 1987).
Ninespine Stickleback / Épinoche à neuf épines
Pungitius pungitius (Linnaeus, 1758)
Taxonomy
Other common names include Tenspine Stickleback, Pinfish, Tiny Burnstickle, Many-spined Stickleback, Kakilahaq, Kakiva, Kakilasak, Kakidlautidlik, Kakilusuk and Kakilishek. The scientific name Pungitius occidentalis (Cuvier in Cuvier and Valenciennes, 1829) has been proposed for this species in eastern North America but has not been widely used (Haglund et al., 1992).
Key Characters
This species is characterised by 6-12 (usually 8-11) dorsal fin spines alternately leaning to the left and right, no obvious, large bony plates on the flank and colour.
Description
Bony plates are present but are small and often 0-8 in freshwater, along the whole flank and onto the caudal peduncle in the sea. The caudal peduncle is long and slender. Dorsal fin soft rays 8-13, anal fin with 1 spine and 6-11 soft rays, pectoral rays 10-11 and pelvic fin with 1 spine and 1 soft ray. The pelvic skeleton is absent in many fish from Wood Buffalo National Park, Alberta and many populations across Canada contain a few fish which have a reduced or absent pelvic skeleton but none are reported from the NCR. There are 11-14 slender gill rakers.
Colour
Overall colour is dark to light green, yellow-green, olive, brown or grey with dark bars, mottles and blotches on the flank and a silvery to yellowish-white belly. Fins are clear. Breeding males are overall jet black and have white to light blue pelvic fins. Fish from Lake Huron have a black ventral patch rather than being black overall.
Size
Reaches 9.0 cm.
Found from all eastern Canada to northern and eastern Alberta, northeastern British Columbia, the Mackenzie valley and all the N.W.T., Baffin, Banks and Victoria islands. In the U.S.A. south to New Jersey on the Atlantic coast, in Great Lakes drainages and around coastal Alaska but not far south on the Pacific coast. Also across northern Eurasia and south to Japan and China.
Origin
This species entered the NCR from a Mississippian refugium as specimens from the Gatineau Valley conform to the Mississippian form as described by McPhail (1963)(Dadswell, 1972; 1974). Its salt-tolerance facilitated dispersal in the Champlain Sea and up the river valleys on the Québec side before sea level fell. McAllister and Coad (1975) recorded fish from Lac Heney, north of the NCR, as having mean values typical of a coastal marine form from the Bering Sea region (lateral plates = 3.67), of a freshwater form (dorsal spines = 9.04) and of an intermediate form (pectoral-pelvic ratio = 1.54, gill rakers = 12.67). NCR populations may have arose from a fusion from both refugia, the freshwater form via the Fossmill outlet of the Great Lakes and the coastal form via the Champlain Sea intrusion. Mandrak and Crossman (1992) suggest both the Beringian and Mississippian refugia are possible sources.
Habitat
Ninespines are found in both fresh and salt waters but usually enter fresh waters to spawn. In lakes it is found down to a maximum of 110 m and it is believed to overwinter in deep water, but it is commonest in shallow bays. It is also common in slow streams and ponds. It is especially abundant in lakes which have a lot of submerged vegetation. Their preferred temperature is 9-10 or 15-16°C, a bimodal distribution.
Age and Growth
Life span may be over 3 years and maturity is attained at 1 year for 90% of males but only 40% of females in Lake Superior. All are mature at age 3. In the Matamek River, Québec life span is 1 year and some months but in Matamek Lake over 2 years. In a tidal creek at Isle Verte, Québec, most fish appear to live about 1 year although some may survive to reach 2 years and some months.
Food
Food comprises aquatic and flying insects, crustaceans, molluscs, worms and eggs and larvae of their own and other fish species. Females feed mostly in the early morning at Isle Verte, Québec. Male diet is more diverse than other sticklebacks because they spend more time away from their nests. This stickleback is commonly eaten by sport fishes.
Reproduction
Spawning takes place in May to July. Individuals with large eggs and breeding colouration are found in Lac Heney, north of the NCR, in June. The male builds a nest off the bottom in dense vegetation, gluing plant fragments together with extruded kidney secretions. In areas without vegetation the nest is built on the bottom and even on turbulent, rocky lake shores. The male defends his nest territory by charges, nipping, biting and chasing. Other encounters involve a slow approach with pelvic spines erect and the head angled slightly down followed by a retreat or the two combatants circling rapidly biting each other's tail. Mouth fighting may also take place in the most energetic fights. Males may drag other males, sculpins or small suckers away by the dorsal or tail fins. The nest is a tunnel open at both ends and about 3-4 cm long. Nests are reported to be open at one end only in Lake Superior, the fish entering and turning around to deposit eggs or sperm. The male courts a female with a complex dance. He angles his body head down, an aggressive position, erecting his spines, twisting his body into a slight s-bend, and zig-zagging towards her. The female assumes a submissive, head up position and follows the male to the nest. The male indicates the nest entrance with his snout, the female pushes past to enter the nest and the male vibrates near her tail to stimulate egg deposition. Each female lays 20-80 eggs and is chased away by the male. He may mate with up to 7 females. Eggs are up to 1.5 mm in diameter and fecundity can reach 136 eggs at Isle Verte, Québec, a tidal creek, compared to only 71 eggs in the freshwater Matamek River system, Québec. Nests at Isle Verte had a mean number of 331 eggs or fry. The male swims through his nest without stopping, but fertilises the eggs. The eggs are fanned and so aerated by the male. A male may build a second nest while still guarding the first one. The young move away from the nest when about 2 weeks old but up till then he catches them in his mouth and spits them back into the nest.
Importance
This stickleback is food for other fishes despite its spines.
Sculpins and bullheads are found principally in marine and fresh waters of the northern hemisphere. There are about 300 species with 63 in Canada but only 1 species in the NCR. A fossil, Myoxocephalus thompsonii (Girard, 1851)(regarded by some authors as a synonym or subspecies of M. quadricornis (Linnaeus, 1758), is recorded for the NCR in a clay nodule from Green Creek (Champagne et al., 1979; McAllister et al., 1987) but extant populations have not been recorded.
Sculpins have a naked body or have scales, plates, prickles or spines. The head is large and is blunt. It often has spines or knobs particularly 1-4 preopercle spines and the body tapers posteriorly. There is a large, dorsally placed eye. The mouth is large but teeth are generally small. There is 1 lateral line. The dorsal fin usually has separate, short spinous and longer soft portions. Pelvic fins (rarely none) have 1 spine (often embedded and hard to detect) and 2-5 soft rays. There are no anal fin spines. The pectoral fin is large and fan-shaped. The caudal is usually rounded or truncate. The hyomandibular bone has a unique lateral process. Adults lack a swimbladder. The males of some species have a penis-like urogenital papilla to deliver sperm internally to females. Eggs are few, large and demersal and may be guarded by the male. Coloration is usually mottled and drab.
Most sculpins are small, shallow water, bottom-living species abundant in tidepools and streams although some are found down to 2000 m. Food is bottom invertebrates such as molluscs, crustaceans, worms, insects and small fishes.
Bergeron and Brousseau (1982) and Bernatchez and Giroux (2000) map Cottus cognatus Richardson, 1836 from the general area of the NCR but this is not confirmed by specimens. Myoxocephalus thompsonii (Girard, 1851) and Cottus ricei (Nelson, 1876) are found in waters just outside the NCR, in Lake Heney, Québec for example. Some literature and museum specimen records of Cottus ricei need verification.
Mottled Sculpin / Chabot tacheté
Cottus bairdii Girard, 1850
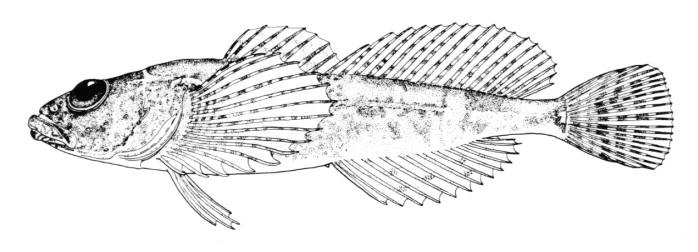
Taxonomy
Other common names include Miller's Thumb, Columbia Sculpin, Muffle-jaw, Spoonhead, Blob, Gudgeon, Freshwater Sculpin, Muddler, Springfish and Lake Sculpin. The subspecies in the NCR is probably C. b. kumlieni (Hoy in Nelson, 1876), the Great Lakes Mottled Sculpin.
Key Characters
This is the only sculpin in the NCR. It has the typical sculpin shape with 3 preopercular spines and 4 pelvic fin rays.
Description
This species has 2 pores on the tip of the chin. There is an incomplete lateral line. Prickles are found only behind the pectoral fins. There are palatine teeth in the roof of the mouth. The last 2 dorsal and anal rays close together and the fourth pelvic fin ray is about three-quarters as long as the longest ray. Usually there are 14-16 (range 12-17) pectoral rays. Caudal peduncle length is less than postorbital length. First dorsal fin spines 6-9, second dorsal rays 15-19 and anal rays 10-16. There are 15-36 lateral line pores. Males have a thin, elongate genital papilla.
Colour
Overall colour light to dark brown or olive with back and sides darkly mottled. The belly is white. There are 2-3 saddles at the second dorsal fin and 2 fainter saddles at the first dorsal fin. There is a dark bar at the base of the caudal fin. Fins are thinly barred brown or dusky. The first dorsal fin has an anterior and a posterior spot. The chin has some speckling. The breeding male develops a dark stripe on the first dorsal fin with a broad orange stripe above it to the edge of the fin. Male overall colouration is darker than the female, being blue-black.
Size
Reaches 17.7 cm.
Found in the Columbia and Milk River drainages of southern British Columbia and Alberta, and in adjacent U.S. states. This distribution is separated from an eastern one stretching from northeastern Labrador, Ungava, western Québec, Ontario and the Great Lakes, to southern Manitoba. In the U.S. it is found south to Georgia and Alabama.
Origin
This species entered the NCR from possibly an Atlantic coastal or Mississippian refugium (Mandrak and Crossman, 1992).
Habitat
This sculpin favours cool streams and lakes over sand or gravel bottoms. They will burrow in the bottom if approached. Depth range is from the shallows down to about 16 m and preferred temperature is 16.6°C.
Age and Growth
Maturity is reached at age 2-3 years and life span is 6 years.
Food
Food is mainly aquatic insects with some crustaceans, worms, rarely macrophytes and small fishes, and fish eggs. However they are not a major predator on eggs of trout and other sport species. Mottled Sculpins feed in the open at night and hide among rocks during the day. Food includes both swimming prey and prey buried in the substrate. The latter can be located by detecting prey movement using the mandible or large lower jaw bone. The sculpin places its lower jaw on the bottom to detect prey vibrations, "hops" towards the stimulus and bites into the sand. The mandible has very large neuromasts which aid in reception of the signal. This ability to detect vibrations may also be used to locate potential predators and in communication among members of a species. They are eaten by Brook Trout and water snakes.
Reproduction
Spawning takes place in spring, mainly in May in Canada at 5-16°C. An eyed egg mass was found in Sawmill Creek, Ottawa on 25 May at a water temperature of 11°C (McAllister and Coad, 1975). Males defend a nest under a rock, and entice females there by a courtship display which involves head shaking and nodding, expanding the gill cover and undulating the body. Knocking sounds are produced by head nods and slaps to the substrate, and drum rolls are produced by a rapid series of knocks followed by a head slap. Spawning takes place at night so sound is very important as a signal in nest defense and possible female choice of males. The male will also bite the female or take her head into his mouth and shake her. Females select larger males to mate with as these have a higher breeding success and favour those with nests under larger rocks. They spend about a week searching for a large male as too long a delay in selection leads to high egg mass failures because of late egg laying. However too large a male will eat the female. Eggs are deposited on the roof of the nest, the female is driven away and the male guards and fans the eggs. Up to 12 females may breed with 1 male but females breed only once per season. Large males succeed in hatching more eggs than small males. Each female may produce up to 635 eggs of up to 3.0 mm diameter and a nest can contain up to 2874 eggs.
Importance
Occasionally caught by anglers and, if they are unfamiliar with sculpins, occasions much surprise from its "ugly" appearance. It may be an important food for Brook Trout in streams.
Centrarchidae - Sunfishes - Achigans et Crapets
Sunfishes, basses, crappies and their relatives are found only in North American freshwaters and have about 32 species. There are 12 species in Canada and 6 in the NCR. The three most abundant species in the Rideau River were Bluegill, Pumpkinseed and Rock Bass in a survey between 1998-2000 (www.rideauvalley.on.ca/programs/rrr/rrr.html, downloaded 15 July 2002).
These fishes are characterised by continuous or notched spiny and soft dorsal fins, the spiny part lower than the soft part, 3 or more anal fin spines, thoracic pelvic fins, 5-7 branchiostegal rays, separate gill membranes, no suborbital shelf and a small, concealed or absent pseudobranch. Body shape is elongate in basses or compressed in sunfishes and crappies. Jaws and mouth cavity bones are variously armed with bands of teeth. There are also conical or molar-like throat or pharyngeal teeth. The swimbladder is not connected to the gut by a duct. Scales are ctenoid, occasionally cycloid.
Hybrids are common among sunfishes and may confuse identifications. Sunfishes are identified by body shape, and by fin ray and scale counts. One unique character used in identification is the "ear flap" or opercular flap, an extension of the upper, rear corner of the gill cover. This flap varies in shape and colour and the body edge may be smooth or crenate. The bony edge is not the obvious rear margin of the flap, which is fleshy, but is an internal structure at varying distances anterior to the rear margin.
Sunfishes build nests, usually a shallow depression excavated by tail sweeps. The male guards the eggs and young. The sunfishes (genus Lepomis) make grunt-like sounds during courtship, an important method of mate recognition. The basses are important sport fishes and a number of sunfish species are used as experimental animals, being easy to maintain in aquaria. Colours of sunfishes rival those of tropical marine fishes and they are used in the aquarium trade. In the NCR they are very common, 79.6% of fish caught in one sample from Mooney's Bay on the Rideau River being three members of this family (Rock Bass, Smallmouth Bass and Black Crappie)(RMOC, 1995a). They have been introduced widely outside their natural range in North America and in other parts of the world.
The record of Lepomis megalotis (Rafinesque, 1820), the Longear Sunfish, from the NCR in Lee et al. (1980) was an error of labeling (Coad, 1985a). However Hubbs et al. (2004) do map this species in the NCR as Lepomis peltastes Cope, 1870, the Northern Longear Sunfish, variously regarded as a synonym, a subspecies or distinct from L. megalotis by authors.
Rock Bass / Crapet de roche
Ambloplites rupestris (Rafinesque, 1817)
Taxonomy
Other common names include Redeye, Goggle Eye, Garguncle and Crapet aux yeux rouges.
Key Characters
This species is identified by having 6 (range 5-7) anal fin spines and 10-13 dorsal fin spines.
Description
Soft dorsal fin rays 10-13, soft anal rays 9-11 and pectoral rays 12-15. Lateral line scales 35-51. Gill rakers 14-19, including about 4 stubby rakers on the upper arch, and 6 long and 6 stubby ones on the lower arch.
Colour
The opercular or "ear flap" is short and not brightly coloured but black with a paler margin. Overall colour above brown with a golden sheen to olive or green with dark saddles and bronze blotches. The belly is silvery to dusky white. Scales below the lateral line have a dark brown spot which line up to form 8-11 or more horizontal rows. The eye is red to orange and can be seen easily from above. The red eye colour fades rapidly once the fish is removed from the water. The dorsal and anal fin spines are darker than the fin membranes. Anal spine tips are white. The pectoral fin is dusky, pale yellow to olive, and other fins are mottled black to brown with white spots or ovals posteriorly on vertical fins. Rapid colour changes from predominantly black to silvery with black blotches are not unusual. Peritoneum colourless. Faber (1984c) illustrates a larval bass.
Size
Attains 43.0 cm and 1.7 kg. The world, all-tackle angling record weighed 1.36 kg and was caught in the York River, Ontario on 1 August 1974 by Peter Gulgin.
Found from southwestern Québec across southern Ontario including upper reaches of James Bay tributaries to Lake of the Woods, southern Manitoba and the Qu'Appelle River of eastern Saskatchewan. In the U.S.A. west of the Appalachian Mountains south to the Gulf Coast. Introduced both east and west of this central American range. Also introduced in England.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Rock Bass are found in groups near rocks and docks in shallow, weedy lake margins, rivers and streams. Turbidity is tolerated. In an Ontario lake, bass were observed to school during the day at 1-7 m in rocky areas. At night they were mostly inactive and settled on rocks or logs. Tagging programmes in southern Lake Ontario show that Rock Bass may disperse 241 km from a release point, although mean distance was only 15.5 km over 238 days. In Lake Erie there is a 35-40 km migration to a spawning site. Stream populations are more sedentary. Their preferred temperature is 20.5°C. A fish kill along extensive stretches of the Rideau River and Canal was reported in spring 1956 (newspaper reports). Several Rock Bass around the Champlain Bridge caught in late June 2005 showed major body wounds, perhaps from predatory birds or possibly eels. Rock Bass were the most abundant species in Campbell's (2001) study of the Mississippi River.
Age and Growth
Life span is up to 18 years in aquaria but most live less than 13 years in nature. Life span in streams is only 5-6 years, much less than in lakes. Growth may be influenced by size of the water body inhabited. Under crowded conditions, stunted populations develop. Acid rain may cause faster growth in some lakes because less young survive to compete for food. Maturity in an Ontario river was attained at 3-4 years, in lakes at 4-9 years. However Rock Bass in the Rideau River showed age groups to 12 years although most were half this age (Setterington, 2004).
Food
Food is a variety of aquatic insects, some surface insects, crustaceans such as crayfish and small fishes such as Yellow Perch, Carp Family members and their own species. Feeding takes place in the evening (1700-2100 hours) and the morning (0930-1200 hours).
Reproduction
Spawning occurs in May to June after a movement into shallow water at 16-21°C in Wisconsin. Spawning starts at about 21°C. A river dwelling population in Ontario (Thames River) began spawning earlier at lower temperatures and lasted longer than in lake populations because of flooding which repeatedly stopped breeding. The season lasted from early May to late July while in Lake Opinicon, Ontario the season is late May to mid-June. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 27 May to 10 June at 13.5-18.5ºC. Older and larger males spawn earliest and thus have more chances to re-nest. Large males build larger nests and a nest was usually completed the day its construction was started. Nests in flowing water were elliptical, about 39-43 cm long. Lake Opinicon fish had peak reproduction at 21-33°C. The male excavates a circular nest up to about 27 cm across in gravel or swampy areas, adjacent to those of many other males, using his pectoral fins in particular as well as the anal fin. Most sunfishes use the tail to sweep debris from the nest site. Some stones are pushed to the nest rim with the open mouth in stream populations. Rooted plants are pulled outside the nest and dropped. Nests are about 1.9 times as large as the male. There is considerable competition for females. Larger males are preferred by females. Spawning males become black. A male successful in attracting a female will remain next to her while she comes to lie on her side so her genital opening is pressed against that of the male. Both fish vibrate and rock back and forth in a head to tail position. A few (3-5) eggs are shed at intervals for an hour or longer. In Lake Opinicon, Ontario about 500 eggs are laid. A nest may contain eggs from more than 1 female and females will deposit eggs in several nests. Up to 11,000 adhesive, orange to golden eggs up to 2.1 mm in diameter are produced. The male defends the eggs until they hatch and fans them with his pectoral fins. He also protects the larvae, which hatch at 4.5-5.5 mm, for 9-10 days until they leave. Males and females can then spawn again and in Lake Opinicon, 24% of males do so. Defense posture includes erect fins and an open mouth.
Importance
A favourite of young anglers since it is easily caught, taking even unbaited hooks. Small (1883) states that it will rise to the fly in the morning and evening in the NCR. It gives a good fight on light tackle and has tasty, white flesh. Rock Bass form a minor part of the commercial catch in Ontario but is combined with crappies in statistics. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, and Ottawa River. As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Pumpkinseed / Crapet-soleil
Lepomis gibbosus (Linnaeus, 1758)




Taxonomy
Other common names include Yellow Sunfish, Common Sunfish, Round Sunfish, Sunny, Punky, Sun Bass, Pond Perch, Bream, Flatfish, Kivvy, Yellow Belly, Tobacco Box, Quiver and Roach. Hybrids with Bluegills, and other sunfishes, are common and may even hybridise with the parents and other hybrids making identification very difficult. Hybrids with Bluegills are known from the Rideau River system in the NCR (RMOC, 2000). The name Pumpkinseed is said to be derived from the body outline resembling these seeds.
Key Characters
This species is characterised by having 9-12 dorsal fin spines, 3 anal fin spines, 35-47 lateral line scales, gill rakers are short (length about equal to width) and knob-shaped or bent, and the short opercular or "ear" flap is black with a white to yellow or orange margin and a red spot at the rear edge.
Description
Second dorsal fin soft rays 10-13, anal soft rays 8-12 and pectoral rays 11-14. Gill rakers 9-13, stubby. The length of the longest raker in adults is less than twice its basal width while in young-of-the-year it is 3-4 times. The pectoral fin reaches the front of the eye when folded forward.
Colour
The back and upper flank are brown or golden green to olive and the lower flank has wavy blue-green lines. The belly is a very distinctive and colourful orange to red-orange. The flank has 7-10 vague bars most evident in females, and is variously spotted olive, orange, red, blue or emerald. The head has alternating, wavy blue-green and orange-brown stripes on its sides and is also spotted with olive, orange or red. The red "ear" flap spot may be orange or yellow and is white in preserved fish. The dorsal spines have a black leading edge. Fin membranes are spotted brown or are black except for the pectorals which are clear to amber. The second dorsal and caudal fin membranes additionally have orange to olive spots. The pelvic fin has a white leading edge. The posterior margins of the second dorsal, anal and caudal fins are blue-green to yellow. Peritoneum silvery. Faber (1985a) illustrates a larval Pumpkinseed.
Size
Reaches 40.0 cm. The world, all-tackle angling record from Mexico, New York in 1985 weighed 0.63 kg but a fish weighing 0.85 kg is recorded from the Notawasaga River, Ontario.
Found in southern New Brunswick, southwestern Québec, southern Ontario but not northern and western Lake Superior drainages, and southeastern Manitoba. Also on Vancouver Island, the Columbia River basin of southern British Columbia and the Oldman River drainage of Alberta as introductions. In the U.S.A. south to Georgia in the east and Ohio in the west but widely introduced elsewhere. Also introduced to Europe.
Origin
This species entered the NCR from a Mississippian or an Atlantic coastal refugium.
Habitat
Pumpkinseeds are extremely common and numerous in weedy bays of lakes, ponds, and slower areas in streams and rivers where the water is clear. They prefer denser vegetation than Bluegills. They are common around docks. The bottom can be sand, gravel, boulders or mud. Their distribution is centred further north than other sunfishes and they generally favour cooler waters. Their preferred temperature is 26°C. In Lac Vert, Québec north of the NCR it is the dominant species of fish in the littoral zone (Beaulieu et al., 1979). During the night they rest on the bottom, in rocky areas or near logs, become pale with prominent bars and do not feed.
Age and Growth
Life span is 10 years (12 in captivity) with maturity attained at age 1-3. Parental males mature about 1 year later than females. Stunting is not uncommon in crowded conditions and has been recorded for 14 years or more in Lac Hertel, Québec and transplants show this is environmental and not genetic. Annual mortality in Lac Vert, Québec north of the NCR is 81% between ages 4 and 7, which compares favourably with estimates from other populations (Beaulieu et al., 1979). These Lac Vert fish matured at age 3+ but were smaller at most ages than those in other studies, possibly due to the lake being oligotrophic and at the northern range limit for the species. Studies of Pumpkinseed growth in lakes suffering from acid rain shows increases because of reduced competition as young Pumpkinseed do not survive well, or conversely decreased growth because of effects on food supplies. In the Plantagenet Reach of the South Nation River an age range of 1-6 years was recorded and 2-7 years in the Spencerville Reach (Lauzon, 2003). Ages as high as 9 years are reported for fish from the Rideau River (Setterington, 2004). Growth in Kettle Island Bay of the Ottawa River was as good as, or superior to, more southerly latitudes (Hanson, 1980).
Food
Food is aquatic and terrestrial insects, crustaceans, worms, snails, salamander larvae, small fishes and some macrophytes. Young-of-the-year Pumpkinseed in the Ottawa River in Kettle Island Bay take midge larvae and water fleas and, at sizes greater than 35 mm length, snails that are crushed and the shells spat out, among a wide variety of other macrobenthic organisms, taken from late morning until just after sunset (Hanson, 1980; Hanson and Qadri, 1984). Feeding in adults involves a wide variety of foods taken during the day, with peaks at dawn and in the late afternoon, by quick darts from a tail-up, head-down position. In Lac Vert, diet consists mainly of fly larvae, water fleas, ostracods and decapod crustaceans but also included winged ants and fish among a wide variety of other organisms (Beaulieu et al., 1979). Pumpkinseeds are eaten by all larger predatory fishes including a wide variety of sport fishes. Even robins have been observed eating this fish, dipping them out of a gap in the ice in a spring seepage into a marshy creek (F. W. Schueler, pers. comm., 11 August 2004).
Reproduction
Spawning occurs form May to August when water temperatures reach values over 12.5°C and daylength exceeds 12 hours. In Dow's Lake the reproductive season starts around 16 May when the first nesting was observed and extends into mid-July (O'Toole et al., 2006). In Pink Lake of Gatineau Park, spawning was observed on a nest site on 10 June 2003 (see pictures above). In Mulvihill Lake in Gatineau Park, no nests were observed on 2 June 2009 but by 5 June several were seen with fish guarding them (see above). Spawning in Kettle Island Bay in the Ottawa River occurred in shallow (<1.0. m) areas cleared of Elodea from the last week of June to the last week of July when temperatures exceeded 20°C (Hanson, 1980; Hanson and Qadri, 1984). Nesting activation began at 16.5°C and lasts 5-6 weeks. Other areas in the NCR have nests with eggs in early to mid-June, hatching in the last week of June. Female Pumpkinseed choose between several males so male courtship is important in increasing the probability of females visiting their nest. Larger males are preferred since they can better defend the brood against predation by snails, for example. The male excavates a nest in shallows near shore using his tail or his mouth for large objects. A nest has a shallow bowl shape about 3.5 times male length, on clay, silt, sand, gravel or rock bottoms in areas with aquatic vegetation. The male and female swim in circles, 11 per minute, nip and bang into each other. The male is vertical but the female inclines so their genital areas are in contact. Eggs and sperm are shed during the circling. Males and females spawn more than once with different partners. Cuckoldry is common where small males rush in to spawn with adults (sneakers) or mimic females (satellites) to achieve the same end. The eggs are amber, about 1.0 mm in diameter and adhesive to vegetation, hard substrate or even small particles. A large female may have as many as 7000 eggs. A nest may contain up to 14,639 eggs from several females. Larvae are 2.5-3.5 mm at hatching. The male guards and fans the eggs and watches over the young for up to 11 days, capturing strays in his mouth and putting them back in the nest. A reproductive phase, however, may occupy on average 10 days, 2 for nesting, 3 for spawning, 4 for brooding and 1 day for vacating the nest. Territory defense around a nest involves puffing out the gill covers, rushes, bites, chases and mouth-fighting. They will nip fingers placed close to the nest. The larvae live in open water beyond the littoral beds of weeds.
Importance
Pumpkinseeds are often the first fish caught by the youngest anglers and will take any live or artificial bait. Even bare hooks are attacked. They are tasty with white, flaky flesh but are seldom eaten by anglers. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. As these limits are apt to change, anglers consuming this fish should consult the most recent version. There is a minor commercial catch in Ontario and Québec which is combined with Bluegills as "sunfishes". Commercial fisheries for these fish above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b). The brilliant colours of this fish make it an excellent aquarium denizen. They may be significant competitors with such sport fish as Yellow Perch as studies in Lake Memphremagog, Québec show a marked reduction in perch growth as Pumpkinseed abundance increased.
Bluegill / Crapet arlequin
Lepomis macrochirus Rafinesque, 1819

Taxonomy
Other common names include Northern Bluegill Sunfish, Common Bluegill, Blue Sunfish, Sun Perch, Bream, Blue Bream, Coppernosed Bream, Pale Sunfish, Bluegill Bream, Bluemouth Sunfish, Roach, Blackear Bream, Chain-sided Sunfish, Dollardee, Strawberry Bass and Crapet à oreilles bleues. Hybrids with Pumpkinseeds are known from the Rideau River system in the NCR (RMOC, 2000).
Key Characters
This species is distinguished by having 9-12, usually 10, dorsal fin spines, 3 anal fin spines, 38-50 lateral line scales, a completely black opercular or "ear" flap which has about the same length as width and has an entire bony edge (not crenate or wavy), and a black blotch at the rear of the dorsal fin base.
Description
Second dorsal fin soft rays 9-13, soft anal rays 8-12, usually 11, and pectoral rays 12-15. Gill rakers 13-16, slender and 4-5 times longer than the basal width. Hybrids with Pumpkinseeds are common in Canada and the hybrids breed with either parental form giving a complete and continuous range of characters between the 2 species. This often makes identification difficult.
Colour
The back and upper flank are green, olive or brownish to almost black with a bluish or purplish iridescence fading to a silver or white belly. The "ear" flap may have a blue anterior edge. Flanks have 5-9 vague, olive, double bands but these can be absent in large fish or fish living in turbid water. The breast is yellow. The sides of the head have a metallic green and blue sheen and the chin and lower operculum are blue. The pectoral fins are transparent and yellowish. The pelvic and anal fins may have a white anterior edge. Young have 9-12 dark flank bars. Breeding males develop a yellowish to copper-orange breast and the pelvic and anal fins become black or dusky. Breeding males show a deep reddish chest when defending the nest and young. A hump develops in front of the dorsal fin. Peritoneum silvery.
Size
Reaches 41.0 cm. Males grow about 25% larger than females. The world, all-tackle angling record from Ketona Lake, Alabama caught in 1950 weighed 2.15 kg.
Found in southwestern Québec, southern Ontario including the Great Lakes but not northern and eastern Lake Superior, although appearing again in its western drainages. In the U.S.A. south to northeastern Mexico and Georgia west of the Appalachian Mountains and from Florida north to Virginia. Also widely introduced throughout North America as well as in Europe and South Africa. The Lac LaPêche population of this species is an introduced one (Chapleau et al., 1997) and this species appear to have become more widespread in the NCR in recent years.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Bluegills are often found in ponds, lakes, streams and rivers where there is little current, shallow water and much vegetation. Bluegills are often in small schools of 10-20 fish. They retire to deeper water in winter, or in summer if the shallows are too hot. While tolerant of some turbidity, they are intolerant of high turbidity and siltation. Their preferred temperature is 30.9°C. In the Long Reach of the Rideau River, this species comprised 41.3% of the species caught during index netting in 2000, far more than any other species (Pumpkinseed was next at 27.3%, followed by Largemouth Bass at 6.7%)(Setterington, 2000). In the Eccolands Reach of the Rideau River this value was 45% for Bluegill with Rock Bass next at 15% (Setterington, 2002).
Age and Growth
Life span is 11 years with older fish in the northern part of the range. Maturity is attained at 2-8 years for males and 3-4 years for females although some populations, especially in the south, mature at 1 year. Growth varies greatly between populations depending on various environmental factors. Stunting is not uncommon in ponds. Bluegills in the Rideau River reach 8 years of age, although most are 5 years or younger (Setterington, 2004).
Food
Food includes aquatic and flying insects, crustaceans, molluscs, worms, fish fry, bryozoans and algae. Growth is better when diet includes some algae. Feeding is greatest at dawn and dusk. Food may be taken in mid-water, on the bottom or at the surface, often using sight to select items. Some Bluegills have been found to contain the external fish louse in their guts. They are "cleaners", picking such parasites off other infected fish. Cleaning has been observed on Largemouth Bass. Bluegills are commonly eaten by other fishes when small.
Reproduction
Spawning peaks in late June to early July in Canada but may extend from May to August in northern populations. In Dow's Lake the reproductive season starts around 5 June when the first colony was observed (O'Toole et al., 2006). Temperatures are usually in the range 19-27°C although Thellen (1994) gives 10°C for the Outaouais. Males excavate a shallow depression about 61 cm across on gravel, sand or mud bottoms using their caudal fins. These shallow water nests are colonial, with up to 50 in an area of radius 21 m at a traditional site. Males compete for access to central nest sites in the colonies, large ones usually winning, and females favour central sites because predation is less. Males defend their nests and eggs. Defense postures involve erected fins, butting and biting but usually stops with a rush at the intruder. A nesting colony of males, females and non-nesting males will mob a large turtle, perhaps to draw attention to its presence or to drive it away. Females arrive on the spawning ground after the males in a large school. Each female selects a male. A spawning pair swims in circles around the nest and then comes to lie side by side with the male vertical and the female inclined so their genital openings touch. Some eggs, about 30, are shed and fertilised and the circling is repeated. The tilting body behaviour of females is called a dip. Males make grunting sounds during courtship perhaps to attract females. A female can have up to 81,104 eggs. The eggs are amber, adhesive and up to 1.4 mm in diameter after water hardening. The eggs are guarded and fanned for 2-3 days, the fry for 3-4 days, after which they disperse as do the males to feed. Nests may be re-used by other males since the spawning season may extend into August. Several females lay eggs in one nest which can have about a quarter million eggs. Adults can have 2-5 brood cycles and reproduce for 2-3 years. In Lake Opinicon, Ontario bluegill colonies spawn on 8 days but these days are spread 5-7 days apart from late May to early July. Small sneaker males rush in to steal fertilisations from adults and satellite males mimic females to also cuckold the large male. DNA evidence shows that about 20% of cuckoldry spawnings are successful. The large male looks after the eggs and young while females, sneakers and satellites leave. Large males are sexually inactive until age 7 in Lake Opinicon, Ontario when they are large enough to defend nests. Sneakers and satellites mature at age 2, first being sneakers and then satellites as they become large enough to mimic females. These cuckolders do not become large, parental males. Apparently selection is acting to maintain this stable system.
Importance
Bluegills are very important sport and commercial fishes in the U.S.A., less so in Canada. In the U.S.A. it is stocked in farm ponds and reservoirs, yielding up to 330 kg/ha/year. They may be caught on flies or live bait and are excellent sport, particularly for the younger angler. Bluegills fight strongly by swimming in circles with the body broadside to the angler giving a disproportionate pull for the size of the fish. There are small commercial catches in Ontario and Québec and the flesh is white, flaky and good eating. Some consider the Bluegill as the tastiest freshwater fish. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and Rideau River. As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Smallmouth Bass / Achigan à petite bouche
Micropterus dolomieu Lacepède, 1802
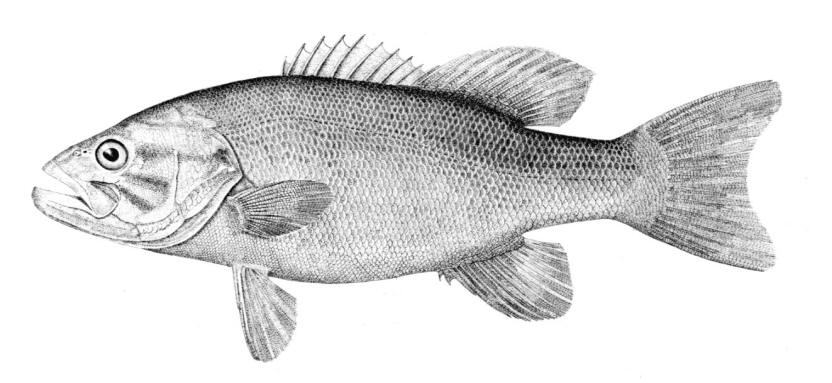




,%20Meech%20Lake,%2013%20June%2003crop.jpg)
Taxonomy
Other common names include Northern Smallmouth, Black, Brown, Gold, Green or Oswego Bass; White, Green or Mountain Trout; Bronzeback, Redeye, Smallie, Jumper and Achigan noir.
Key Characters
This species is distinguished by having 67-81 lateral line scales, the upper jaw not reaching back beyond the eye and the pelvic fins are joined by a membrane.
Description
There are scales on the soft dorsal and anal fin bases. Dorsal fin spines 9-11, soft dorsal rays 12-15, anal fin with 3 spines and 10-12 soft rays. Pectoral rays 13-18. Gill rakers 6-11, 6-8 developed.
Colour
The back and upper flank are dark brown, olive or green, the flanks are lighter, yellowish with some slatey areas and with a more golden colour, and the belly is cream to white, with some dusky pigment. Flanks have 8-16 thin bars which may only be weakly developed or broken up. The eye and snout have 3 dark bars radiating backwards. The eye is orange to red. Fins are dusky with some heavier black pigment along the rays. The pectoral fin is mostly transparent. Young have strong flank bars and a characteristic yellow to orange bar at the caudal fin base with a black bar on the fin and white to yellow fin tips. The fry are conspicuously jet black. Peritoneum silvery. Faber (1984c) illustrates a larval bass.
Size
Attains 68.6 cm total length and 6.4 kg. The world, all-tackle angling record from Dale Hollow Lake, Kentucky in 1955 weighed 5.41 kg. The Ontario record as of the year 2000 weighed 4.5 kg. Small (1883) records a 5 lbs 8¾ oz (2.5 kg) fish from Lake Bernard, Taylor Lake, Gatineau Park has fish to 49.7 cm (Pluritec Ltée, 1982), the Ottawa River has bass to at least 2.24 kg (http://backwaterbass.homestead.com/ottawabbass2002~ns4.html, downloaded 9 June 2003), the Rideau River has bass to 19 inches and 4.5 lbs near Kars (www.fish-hawk.net, downloaded 16 June 2003), one weighing about 5 lbs was taken from the Rideau Canal at the Bronson Street bridge (P. Minns, pers. comm., 7 June 2004), and the Lièvre River has trophies to 2.25 kg (Campeau, 2002). A fish caught in the upper Madawaska River, outside the NCR but draining to it, was estimated to weigh 12 lbs 4 oz (5.68 kg) and was 22 inches (55.9 cm) long but had been gutted and filleted by the angler (Ottawa Citizen, 19 July 2007, p. A1 and A11, with photograph). The guts, head and fillets were retrieved and weighed.
Found from southern Nova Scotia and New Brunswick, through southwestern Québec, the southern half of Ontario including all the Great Lakes to southeast Manitoba. Also introduced in these provinces and in central Saskatchewan, central Alberta, southeastern British Columbia and southern Vancouver Island. Widely introduced in North America, Europe, the former U.S.S.R. and even Africa. They were introduced into Meach Lake in about 1908 when 28 fingerlings were released (Dymond, 1939). They have been stocked below the rapids near Britannia in the Ottawa River in September 1941, comprising 1000 fingerlings 4 inches long and 100 adults 12-14 inches long (newspaper reports). They have been stocked in the Jock River at Richmond in 1955 (newspaper reports).
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Smallmouth Bass are found in the clear shallows of lakes and slow rivers. The bottom is mud, rock, boulders or sand. They are often located near rocks and logs. They seem to prefer temperatures in the 20s°C, cooler than Largemouth Bass, and so retreat to deeper water in summer. Another report gives 30.3°C as the preferred temperature, almost exactly the same as Largemouth Bass. They are inactive in winter. A fish kill along extensive stretches of the Rideau River and Canal was reported in spring 1956 for black bass, presumably this species or its relative (newspaper reports).
Age and Growth
Life span is 18 years. Age groups up to 14 years are recorded for the Mississippi Lake and Mississippi River at Carleton Place (Kerr, 1999c) and 12+ years in Taylor Lake, Gatineau Park (Pluritec Ltée, 1982). Females mature at 4-6 years and males at 3-5 years. Growth varies with latitude but also between populations within lakes such as Simcoe, Huron and Ontario and between oligotrophic Laurentide lakes and the richer, eutrophic waters of the Montréal plain. Bass introduced into Meach Lake in the NCR reduced the minnow population, and the numbers and weight of bass are limited by food scarcity. In the Plantagenet Reach of the South Nation River an age range of 1-6 years was recorded (Lauzon, 2003).
Food
Food is aquatic and terrestrial insects, crustaceans (particularly crayfish) and a wide variety of fishes. Frogs and fish eggs are also eaten. Crayfish are predominant in the diet of many bass populations but habitat and availability dictate the foods eaten. Various fishes and turtles eat the smaller bass, including Lake Trout in the NCR (Séguin and Veilleux, 1970).
Reproduction
Spawning occurs from May to July at about 12-24°C. In Dow's Lake the reproductive season starts around 9 May when water temperatures reach 15ºC (O'Toole et al., 2006). In lakes McArthur and Grand in the Blanche River drainage in Québec, larvae were observed in June (Séguin and Veilleux, 1970). The male excavates a nest up to 1.8 m across, but usually 60 cm, in quiet water at depths down to 6.1 m but usually about 1 m. The bottom is usually sand, gravel or rubble. Nest construction takes 4-48 hours usually and several may be built until one is deemed adequate. The bass positions itself head up in the water and lashes the tail to sweep debris from a site about twice its body length. The nest is usually close to a rock, log or rarely vegetation which afford some protection. Strong winds are particularly detrimental to nesting success. The nest site or its general area is returned to in subsequent years, up to 32% of spawners doing so in an Ontario river. Males defend the nest by displays and nipping. A female approaches the shallows from deeper water and a male will attempt to drive her to the nest. She usually retreats and the process is repeated until the female gradually stays longer with the male. The female becomes mottled once she settles over the nest. The male may nudge and gently bite the female. A spawning pair swim about the nest for 25-45 seconds before entering it and settling on the bottom. The male remains vertical but the female inclines at an angle of 45° so their genital areas come into contact. Eggs and sperm are shed in about 4-10 seconds but the process is repeated over 2 hours. The spawning period at any one location usually lasts 6-18 days and most spawning takes place in the evening. Eggs are pale yellow to amber, 3.5 mm in maximum diameter and number up to 20,825 per female. The eggs adhere to stones in the nest. Larvae are 4.5-5.5 mm at hatching. The male guards the eggs and school of young and fans the developing eggs. The male guard erects his dorsal fin spines, rigidly extends the pectoral fins and charges at any intruder. Schools of young fish disperse after 19-28 days and may be guarded this long, herded by the male. Females may spawn with other males and the male may spawn with at least 3 females. Male bass may provide care for the eggs of Longnose Gar which may be shed at night when bass are inactive. While gar benefit from the bass guarding their eggs, the bass also benefits by having large, dispersed gar eggs in its nest which may be seized preferentially by predators dashing into the nest while the male is distracted. Common Shiners may also spawn in bass nests.
Importance
This bass is a famous sport fish which has an extensive following and literature. Hooked fish fight strongly and leap, "inch for inch and pound for pound, the gamest fish that swims". It is one of the top 5 sport fishes in eastern Canada and is caught on live baits such as minnows, worms, frogs, and crayfish, on artificial lures or by fly fishing. The flesh is white, flaky and excellent eating. It may be frozen easily for later eating. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Dymond (1939) records commercial catches from 1875 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 20,998 kg in the Ottawa River from Carillon to Pontiac (Québec), the highest recorded. On the Ontario side the highest catch was recorded for Prescott and Carleton counties in 1896 at 8376 kg. A. Martel (personal communication, 2002) considers that the introduction of bass into Gatineau Park lakes, as well as depleting cyprinid populations, also led to a decline in clams that used the cyprinids in their reproductive cycle (clams produce larvae, called glochidia, that attach to a specific fish host and are thus dispersed).
Bony protrusions through the flesh seen in fish from the urban/suburban Rideau River, the Mississippi River near Carleton Place and the Ottawa River downstream of the Green Creek sewage outfall are ribs, a condition probably caused by the parasite Myxobolus cerebralis (Hopkins, 1991d; Belisle, 1999; Kerr, 1999c; RMOC, 2000; Wachelka et al., 2000).
Largemouth Bass / Achigan à grande bouche
Micropterus salmoides (Lacepède, 1802)
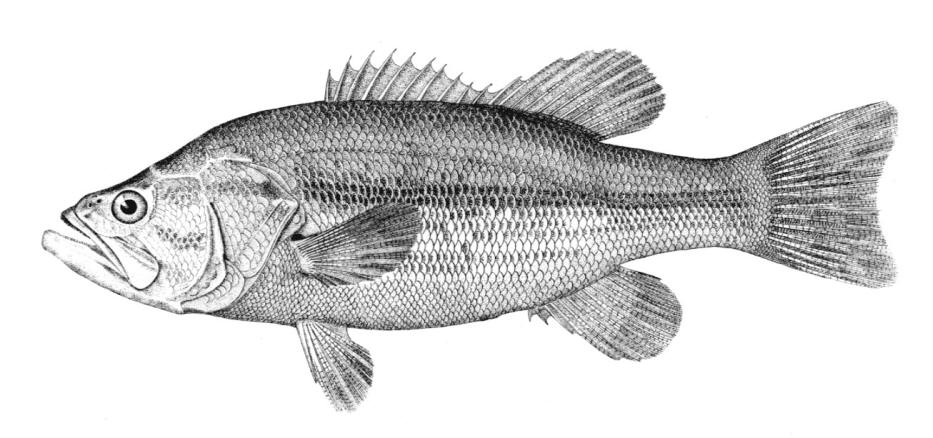


Taxonomy
Other common names include Northern Largemouth, Black, Green, Bigmouth, Jumper, Mossback, Oswego, Cow, Lake, Straw, River and Mud Bass; Line Side and Green Trout.
Key Characters
This species is distinguished by having 9-11 dorsal fin spines, 57-77 (usually less than 70 in Canada) lateral line scales, the upper jaw extends back beyond the eye and the pelvic fins are not joined by a membrane.
Description
There are no scales on the bases of the soft dorsal and anal fins. Second dorsal fin soft rays 11-14, anal fin with 3 spines and 10-12 soft rays and pectoral rays 13-17.Gill rakers 6-10, 7-9 developed and rudimentary on the upper arch.
Colour
The back is bright green to olive or dark green, the flanks dark to light green or sometimes golden green, and the belly is yellowish to white. The flank has a black mid-lateral stripe sometimes extending to the snout, particularly prominent in young but breaking up irregularly into blotches or even absent in adults. The upper and lower flank are irregularly spotted with dark brown, sometimes forming short stripes. The eye is golden brown. The inside of the mouth is white. Dark bars radiate from the eye and snout backwards. Pectoral fins are mostly clear or lightly pigmented. Other fins are green to olive or dusky. Young are similar to adults but have a broad black margin to the caudal fin and more mottled upper flank. Rare, golden forms of this bass occur in nature, similar to a Goldfish in colour. Peritoneum silvery.
Size
Reaches 97.0 cm and possibly 11.37 kg. The world, all-tackle angling record from Montgomery Lake, Georgia in 1932 weighed 10.09 kg. The Ontario record as of the year 2000 weighed 4.7 kg. A 5 lbs 10 oz (2.55 kg) fish is reported from the Ottawa River at Arnprior (www.bassresource.com/html/photowall5.html, downloaded 9 June 2003), a 6 lbs (2.72 kg) fish is reported from Rockland on the Ottawa River (www.fish-hawk.net, downloaded 20 May 2003), a 6.61 lb "bass", presumably this species, was caught at Rockland in the Ottawa River 2 August 2003 and "bass" of 4.45 lbs and 5.46 lbs have been taken at Taylor Park on the Rideau River on 5 July 2003 and 28 September 2003 (http://baoo.canadianangler.ca, downloaded 17 May 2004).
Found from southwestern Québec, southern Ontario and through all the Great Lakes to southeastern Manitoba. Also introduced to southern British Columbia, Alberta and Saskatchewan. In the U.S.A. south to the Gulf states and widely introduced. Introduced to Europe, Africa, South America and Southeast Asia. They have been stocked in the Jock River at Richmond in 1955 (newspaper reports).
Origin
This species entered the NCR from a Mississippian refugium or possibly an Atlantic coastal refugium (Mandrak and Crossman, 1992).
Habitat
Largemouths are found in still, shallow areas of lakes and some large river bays, usually at depths less than 6 m. Small schools of 5-10 fish are common. It is less common than its relative, the Smallmouth Bass, in the NCR. Largemouth are said to be more common upriver from Manotick in the Rideau River and Canal system than downriver (P. Minns, pers. comm., 7 June 2004). Unlike the Smallmouth Bass, it favours heavy, aquatic vegetation and submerged logs, and it can survive higher temperatures, up to 38°C although it then rests in shade. Preferred temperature is 30.2°C. Young bass divide into two sub-populations in summer, one inshore and the other offshore, and this may persist through life with some mixing in spring. In the nutrient-poor, Precambrian Shield lakes of Ontario, the size of bass populations is limited by the extent of warm, weedy shallows. Aquatic macrophytes are essential for the survival of fingerlings.This bass is found on the bottom in winter and may then be caught by ice fishermen unlike the inactive Smallmouth Bass. Experimental studies show Largemouth Bass to be quiescent and non-feeding at 3°C but able to react well to a stimulus.
Age and Growth
Life span is 23 years with maturity attained at 3-5 years for females and 3-4 years for males in Canada (8 months in Cuba). Age groups up to 11 years are recorded for the Mississippi Lake and Mississippi River at Carleton Place (Kerr, 1999c) and the Rideau River (Setterington, 2004). Growth varies with locality, generally slower in the north where fish live longer. Some populations become stunted through overcrowding. Growth in Kettle Island Bay of the Ottawa River was as good as, or superior to, more southerly latitudes (Hanson, 1980).
Food
Food is a wide variety of fishes, crayfish, molluscs, frogs and large insects, with smaller insects and plankton when small. Some are recorded as having eaten leeches, salamanders, snakes, turtles and mice. Largemouths are sight feeders, taking prey at the surface around dusk and dawn and in mid-water or on the bottom during the day. They rest on the bottom at night. This species is cannibalistic. Bass catch individual minnows quite easily but were confused by schools of 8 or more fish, unless one or two members are distinctive in some way. Food items may be pursued or simply sucked in while lying in wait. Recorded foods for young bass in Kettle Island Bay of the Ottawa River are Silvery Minnows, Emerald Shiners, young-of-the-year Brown Bullheads young-of-the-year Rock Bass and some Iowa Darters, taken mostly in late morning and early afternoon (Hanson, 1980). A switch from zooplankton to fish occurred when these fish were 35 mm long. Tadpoles entered the diet at 66 mm at the end of July and were the most important food in August and the end of September.
Reproduction
Spawning occurs from May to August once water temperatures reach 17°C or warmer. In Dow's Lake the reproductive season starts around 9 May when water temperatures reach 15ºC (O'Toole et al., 2006). The male sweeps out a circular nest, a little before this temperature is attained, on gravel, sand or even mud among reeds, stumps, or water lilies. The nest bed often has roots of the vegetation exposed or other hard materials for the eggs to adhere to. The nest is up to 1 m across and 30 cm deep, about twice the bass's body length. Males are very aggressive and defend territory up to 7 m from the nest. Courtship involves parallel swimming, nudges, nips and bites. The male assumes an upright position in the nest with the female at a 45° angle so their genital areas are in contact. Eggs are scattered over the nest and even outside it. Each female can have up to 145,000, yellow and adhesive eggs with a maximum diameter of 2.0 mm. Females may spawn with several males, depositing portions of her egg complement in each nest. The male guards and fans the eggs which hatch in 3-7 days. The male also guards the pale green, free-swimming fry for as long as a month. The large swarm of young spread out as time goes by, keeping the male busy to defend them all, until they disperse. In Kettle Island Bay of the Ottawa River, bass eggs hatched on 6 June at 20ºC and fry averaged 5.0 mm in length (Hanson, 1980). Some males were still guarding fry on 29 June and 29 July so the spawning period was about 6 weeks. Golden Shiner eggs were mixed in with those of bass as this minnow shares the nest.
Importance
This bass was once commercially important in Canada but is now reserved for anglers. It is a major sport fish in eastern Canada taken with minnows, frogs, worms, both live and plastic, surface plugs and other lures. In Ontario competitive fishing tournaments "basses" ranked first among species sought at 37.4% of events (Kerr, 1999d). Bass strike strongly and fight well. Swimming ability decreases as temperature falls so anglers may find bass put up less of a fight early and late in the year. Their successful capture is made more difficult by their habitat of dense vegetation and logs. They are reputed to be very intelligent, able to distinguish a lure from the real thing after one encounter. Some "fished out" lakes are said to be really full of fish familiar with all the usual lures. Size limits are imposed in many waters to preserve the stocks. Studies on return of hooked juveniles show that most survive, with significant mortality only in fish hooked deep in the oesophagus. In the U.S.A. this fish is stocked in ponds and reservoirs for sport and for food and is probably that country's most important freshwater game fish. Warm temperatures needed for spawning and low winter oxygen levels are often limiting factors to the spread of this fish in Canada.
The flesh is flaky, white and excellent eating. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River and Rideau River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Black Crappie / Marigane noire
Pomoxis nigromaculatus (Lesueur, 1829)
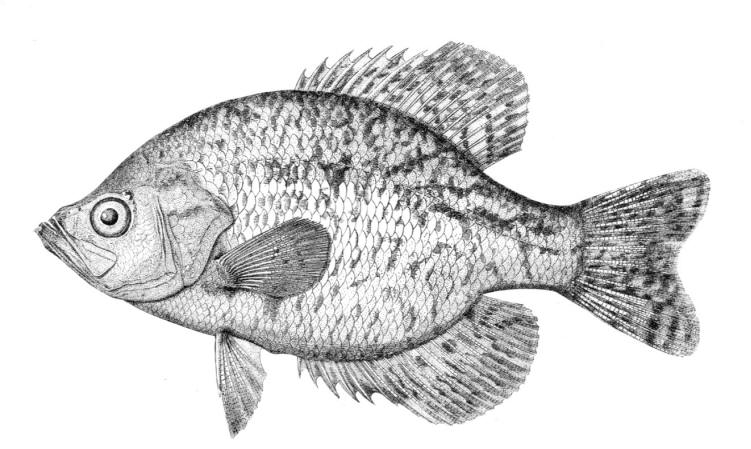

Taxonomy
Other common names include Calico, Crapet Calico; Strawberry, Speckled, Silver, Banklick, Moon, Butter, Straw, Grass or Oswego Bass; Shiner, Moonfish, Lamplighter, Papermouth, Bitterhead, Razorback, Bachelor Perch, Tinmouth, Barfish, Specks, Slab and Mason Perch.
Key Characters
This species is characterised by usually 7-8 (range 6-9) dorsal fin spines, an anal fin base slightly longer than that of the dorsal fins, and flanks irregularly blotched with black.
Description
Second dorsal fin soft rays 14-16, anal fin with 6-7 spines and 16-19 soft rays and pectoral rays 13-15. Lateral line scales 31-44. Gill rakers slender, numbering 22-23 on the lower arch and 5-6 on the upper arch.
Colour
The overall colour is silvery with some iridescent green, the back is olive, brown or dark green, the flanks silvery green with dense black blotching, and the belly is silvery-white. Eye yellow-brown. The dorsal, anal and caudal fins have black vermiculations or "worm tracks" surrounding yellow to pale green spots or ocelli. Pectoral fins are dusky. Pelvic fins have black tips and white leading edges and are generally opaque. Breeding males become much darker and more iridescent on the head and breast. Peritoneum silvery. Faber (1984c) illustrates a larval crappie.
Size
Reaches 48.9 cm and 2.3 kg. The world, all-tackle angling record from Kerr Lake, Virginia was caught in 1981 and weighed 2.05 kg. The Ontario record from Lake Erie, as of the year 2003, weighed 1.7 kg and measured 43 cm.
Found from extreme southwestern Québec in the St. Lawrence, Richelieu and Ottawa river basins, southern Ontario including all the Great Lakes, and southern Manitoba. Also introduced to the lower Fraser River in British Columbia. In the U.S.A. south to Florida and Texas and west to Montana but widely introduced.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
This schooling crappie is found in still waters of lakes and ponds or slow flowing, large rivers where there is abundant plant material or other cover. Areas with access to deeper water in summer and winter are preferred. It grows more slowly in turbid conditions than in clear waters. Preferred temperature is 21.7°C.
Age and Growth
Life span is 13 years with maturity attained at 1-3 years. Growth varies with the habitat and with latitude to some degree. However Ottawa River populations at the northern range limit had growth comparable to other populations, reaching an age of 8+ years, maturing at age 2+ and having a condition factor higher than most other populations (Hanson, 1980; Hanson and Qadri, 1980a). In the Plantagenet Reach of the South Nation River an age range of 2-7 years was recorded (Lauzon, 2003) and fish up to 7 years are reported from the Rideau River (Setterington, 2004).
Food
Food includes plankton, aquatic insects and fish fry when young. In the Ottawa River in Kettle Island Bay, young-of-the-year Black Crappie feed predominately on copepods and water fleas from June to September, switching to amphipods in October as they grew. Fish fry are very important at 15-30 mm length (88.6% of stomach contents at 26 mm), and the fly larva Chaoborus for crappie greater than 50 mm length, Snails and clams are fed on at 38 mm (Hanson, 1980; Hanson and Qadri, 1979; 1983). Even adults may rely principally on water fleas for food, taken by filter feeding in midwater using the numerous gill rakers, but fish become increasingly important as black crappies grow. In the Ottawa River fly larvae are the dominant food of Black Crappies 61-110 mm in total length while crappies larger than 110 mm fed on fish such as Golden Shiners, Eastern Silvery Minnows, Emerald Shiners, Pumpkinseeds, young Black Crappie and Rainbow Smelt. Stone flies were also important diet items for fish 161-210 and 261-315 mm long (Hanson and Qadri, 1980a). Stunting is not uncommon where forage fish are rare. Feeding continues through winter, an unusual habit in a sunfish. The Black Crappie is a predator on the young of sport fishes and may affect population numbers significantly. Peak feeding is between early evening and the early morning hours and in the morning around dawn. Young are diurnal and feed mostly in littoral mid-waters in the morning with a slight peak near sunset in Kettle Island Bay (Hanson, 1980).
Reproduction
Spawning occurs in May to June at 13-23°C in Ontario. In the Ottawa River, Black Crappie spawn in the first week of June, a shorter spawning period than other populations, in water 0.25-6.1 m deep at 18-20°C (Hanson, 1980; Hanson and Qadri, 1980a). Comtois et al. (2006), in contrast, report fish in reproductive stage V (spawning) in the lower Gatineau River from 3 May to 13 May at 9-12.5ºC. Males clean a rounded area in vegetated shallows or under banks. Each nest can be up to 38.1 cm across and nests occur in colonies, about 2 m apart. Each male guards his nest, fans the eggs and protects newly hatched young. Larvae are 4.0-5.0 mm at hatching. Each female spawns with more than 1 male, depositing white adhesive eggs about 0.9 mm in diameter. A female can carry up to 188,000 eggs.
Importance
This species enters into commercial catches. The flesh is white and flaky and said to be as good as the Walleye. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Rideau River, South Nation River and Ottawa River. As these limits are apt to change, anglers consuming this fish should consult the most recent version. Sport fishermen catch them on baited hooks in both summer and winter, the latter catches being under ice. Baits include minnows and insect larvae. Flies, spinners and jigs are also used.
Percidae - Perches - Perches et Dards
Perches, darters, pike-perches and their relatives are found in freshwaters across the northern hemisphere. There are about 162 species total with 148 in North America. The Canadian members number 16, including 1 exotic species from Europe. There are 9 species in the NCR.
Perches have 2 dorsal fins, the first spiny (6-9 spines) and the second soft rayed, which are usually separate or only slightly joined. The anal fin has only 1-2 spines (rather than 3 as in related families). The pelvic fins are under the pectorals (thoracic) and have 1 spine and 5 soft rays. There are 5-8 branchiostegal rays and the branchiostegal membrane is not attached to the isthmus. Scales are ctenoid. Teeth may be long and sharp or small and in bands. The operculum has a sharp spine. There are 2 kinds of perches - large species with compressed bodies and a swimbladder, and derived from them small species with depressed bodies and reduced or absent swimbladders. This condition is believed to have arisen twice within the family. In North America the larger species include the Yellow Perch, Sauger and Walleye and the smaller species are the darters. In darters the mouth is small, not extending back past the anterior eye margin, and the lower edges of the preopercle bone on the side of the head are smooth.
Perches are found in warm southern waters to subarctic ones, in both flowing and still water. Some larger species are commercially important in Canada while smaller species make attractive aquarium fishes. The small darters rival coral reef fishes for colour when in breeding condition.
Darters are sensitive to environmental change and are useful indicators of the health of aquatic ecosystems. The Sauger, Walleye and Yellow Perch are very important species in sport fisheries in Canada. Walleye and Sauger are the first two sport fishes in order of priority for anglers in the Ottawa River below Ottawa (Hopkins, 2000).
Perches have a variety of reproductive strategies which include broadcasting, stranding, burying, attaching, clumping and clustering. During the breeding season tubercles develop, particularly on the male. These may be on the body, fins or head and are used to maintain contact and enhance grip between males and females during the spawning act. Darters in the genus Percina have enlarged and strongly toothed scales between the pelvic fin bases and along the belly mid-line in males. The male probably uses these scales during spawning to stimulate the female. Female darters often have enlarged genital papillae and in those species which lay eggs in gravel or plants the papilla is an elongate tube. Some darter males have egg-shaped knobs on the dorsal or paired fins and these are used to attract females to a nest site. Females are thought to be more likely to spawn with successful males, which already have eggs or convincing mimics of eggs, in their nests.
Certain darters, such as the Iowa and Johnny Darters, have large secretory cells in their skin. These release a chemical alarm substance into the water when damaged in a predator's attack. The chemical signals other darters to "freeze", hopefully avoiding detection by the predator. Studies in southern Ontario indicate that darters are not often food for other fishes, despite their small size, but they are an important food for common mergansers during the spring migration of this bird.
Iowa Darter / Dard à ventre jaune
Etheostoma exile (Girard, 1859)
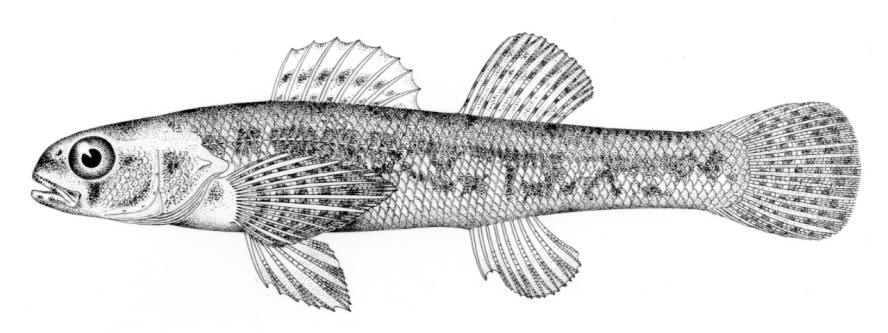

Taxonomy
Other common names include Yellowbelly, Red-sided Darter, Weed Darter and Dard d'herbe.
Key Characters
This species is distinguished from other darters by having the anal fin smaller than the soft dorsal fin, the snout and upper lip joined (i.e. premaxillaries not protractile), belly scaled, cheeks and opercles scaled (may be obscured by skin), dorsal fin spines longer than eye diameter, 7-12 (usually 8-9), soft rays 9-13 (usually 10-11), and lateral scales 45-69 (usually 48-60) with 18-35 pored.
Description
Anal fin with 1-2 spines and 6-9 soft rays. Pectoral rays 12-14. Gill rakers short, numbering 8-10. The opercles are scaled. Males have larger first dorsal and anal fins than females.
Colour
Overall colour is olive-brown to dark brown fading to cream or yellowish on the belly. There are bars radiating from the eye. The back has 7-12 vague saddles and the mid-flank 9-14 short bars or squarish blotches. The caudal fin is barred and there is a basal spot, flanked by a spot above and sometimes one below. Breeding males have a first dorsal fin with a blue basal band or a series of blue spots each surrounded by a clear halo, a transparent band, an orange-red band and a deep blue or blue-green marginal band. The bars on the flank are dark blue or blue-green and the spaces between them yellow, orange or brick red. The belly is yellow, orange or red. The pectoral and anal fins are orange-yellow or yellow-red. Simon and Faber (1987) give a description of pigmentation of larvae from Lake Heney, north of the NCR.
Size
Reaches 7.5 cm.
Found from eastern and southern Québec westward through the Great Lakes basin and tributaries of Hudson Bay to Alberta and across the northern U.S.A. paralleling this distribution. Also reported from northern Alberta.
Origin
This species entered the NCR from a Mississippian refugium or possibly a Missourian refugium (Mandrak and Crossman, 1992).
Habitat
The habitat of this darter is weedy areas of lakes, ponds, marshes, bogs, streams and rivers with clear water. Water current is slow to still and there is usually vegetation. At night it hides in crevices, holes and under logs and branches. Iowa Darters have an alarm substance in their skin. Damage to the skin as in a predator's attack releases the alarm substance which other darters can detect, initiating predator avoidance behaviour. The preferred temperature range is 12-25°C.
Age and Growth
Iowa darters may live 4 years and females are larger than males. Maturity is attained at 1 year.
Food
Food is aquatic insects and crustaceans, snails and fish eggs. Midwater food items are taken, unusual in darter.
Reproduction
Spawning occurs in May-June in Canada preferentially on roots, usually those under banks, or on sand or pebbles in shallow wate. Males have territories and females spawn with several males. The male lies alongside or over the female with his tail depressed and paralleling hers and his pelvic fins over her dorsal fin. In a stream tributary to Lac LaPêche, a male and a female or two males and a female, vibrated simultaneously and eggs fell on leaves, twigs or algae-covered logs or in crannies or gravel in clear water 6-8 inches (15-20 cm) deep with moderate current. This occurred at the end of May and the beginning of June at 9°C (McAllister, 1971). Eggs are laid in groups of 3-7 and are up to 1.3 mm in diameter. Eggs attach to roots and weeds. The male continues to guard his territory but does not care for the eggs directly. Large females may lay up to 2048 eggs. Eggs hatch in 9-10 days at 13-16°C. Larvae are 3.0-4.0 mm long at hatching.
Importance
None although it is common in eastern Canada and is probably food for other fish species.
Fantail Darter / Dard barré
Etheostoma flabellare Rafinesque, 1819
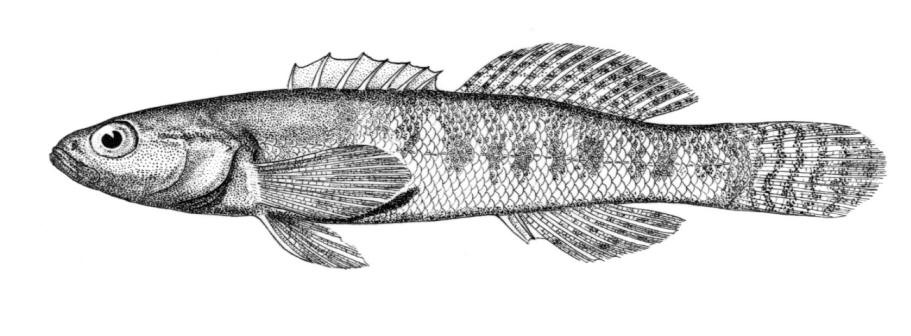
Taxonomy
Other common names include Striped Fantail Darter and Barred Fantail Darter.
Key Characters
This species is distinguished from other darters in Canada by having the anal fin smaller than the soft dorsal fin, the snout and upper lip joined (i.e. premaxillaries not protractile), belly is scaled, dorsal fin spines short, about eye diameter or less, 5-10, usually 7-8, soft rays 10-15, lateral scales 38-60 with 11-59 pored.
Description
Anal fin with 2 spines and 6-10 soft rays. Pectoral rays 10-14. Gill rakers short, numbering 10-12. The nape, cheek, opercle and breast lack scales.
Colour
Overall colour is olive-brown with 5-15 dark flank bars or sometimes thin stripes, or both on a yellow-brown background. There are about 8-10 dark brown to black saddles. Belly cream, yellowish, yellow-orange or dusky. The second dorsal fin has 6 narrow stripes and the caudal fin has 4-7 wavy, dark bars. Bars are present before and behind the eye but the one below the eye is weak to absent. Spawning males develop white to yellow-orange, fleshy knobs at the tips of the dorsal fin spines. The base of the fin becomes thickened, perhaps to produce an anti-bacterial and anti-fungal mucus. Cheeks are olive-green and the rest of the head black or dusky. The breast is dusky. The spiny dorsal fin develops basal and marginal orange stripes and the flanks are yellowish.
Size
Attains 8.6 cm.
Found from southern Québec and eastern New York across the southern Great Lakes to Iowa and Kansas and south to Louisiana and Mississippi. In Canada it is recorded from the upper St. Lawrence River drainage in southernmost Québec, along the lower Ottawa River and tributaries of Lakes Ontario, Erie and southern Lake Huron.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
The preferred habitat is streams with slow to moderate or even fast current. Riffles and raceways are used by this species. Deep areas are used for a winter retreat and gravel and boulder bottoms for spawning. Also found less frequently in lakes. The preferred temperature is 22.4°C.
Age and Growth
Males live longer than females and are larger. Males usually reach 4 years and females 3 years of age, but these fish are mature at 1-2 years of age. Maximum age is 5 years.
Food
Food is aquatic insects, such as net-spinning caddisflies, blackflies and stoneflies, and crustaceans taken from between and under rocks in the morning and evening. Some molluscs are taken occasionally. The fantail spends most of its time on the bottom in crevices waiting for prey. Food items can be almost as long as the fish.
Reproduction
Males set up a territory in spring (April to June) under a rock, cleaning the rock surface with the fleshy knobs of the dorsal fin spines. The male becomes very dark when chasing away intruders. Males threatened by other fishes "freeze" for 2-3 minutes and then resume normal activity. Females enter male territories, poking their heads under rocks and darting from rock to rock. Courtship involves leading a female to the rock cavity with circling, figure-of-eight swimming, nudging and prodding, and entry and exit from the cavity until the female turns upside down. The swimming patterns are repeated with the male right side up and head to tail with the female. The female eventually quivers and lays 1-3 eggs which stick to the rock, the male flips over to quiver and fertilise the eggs and then flips back again. Up to 45 eggs are laid and fertilised in this manner with the female inverted for up to 2 hours. The cavity under the rock is narrow enough so that the female's dorsal fin contacts the bottom and offers some support. The male may mate with several females and the female with up to 5 males in each spawning season. He guards the eggs and brushes them with his dorsal fin knobs. It is probable that the male is smearing mucus on the eggs to prevent bacterial and fungal attack. The number of eggs in a nest can vary from 8 to 562. The eggs are up to 2.7 mm in diameter. In the NCR a ripe female had eggs 1.6 mm in diameter on 20 May.
Importance
This darter makes an excellent aquarium fish.
Johnny Darter / Raseux-de-terre noir
Etheostoma nigrum Rafinesque, 1820
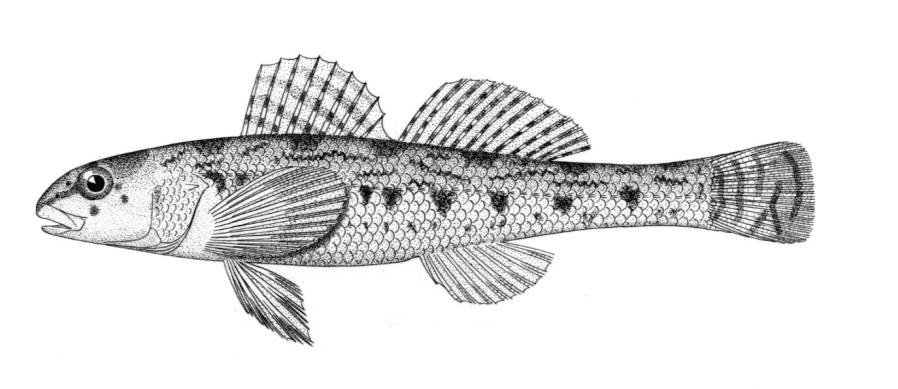
Taxonomy
Another common names is Central Johnny Darter. Hybrids with the Tessellated Darter are reported in the NCR (McAllister et al., 1972).
Key Characters
This darter is recognised by the small mouth not extending beyond the anterior eye margin, a smooth edge to the preopercle bone on the side of the head, an anal fin smaller than the soft dorsal fin, belly scales present or absent but no enlarged scales in mid-line, premaxillaries protractile, i.e. a deep groove separates the upper lip from the snout, 1 thin anal spine, 1-11, usually 9 or less, pores in the preoperculomandibular canal (the head sensory canal running from the lower jaw onto the preopercle bone), and 9-15, usually 12 or less, dorsal fin soft rays.
Description
Dorsal fin spines 6-10, anal soft rays 6-10. Pectoral fin rays 10-14. Lateral line scales 35-59. There are 8-11 short gill rakers. The breast, cheek and nape are usually scaleless.
Colour
Overall colour is pale brown or sandy with yellowish or greenish tints depending on habitat. The back has about 4-7 dark brown saddles. The flanks have 7-12 distinctive X-shaped pigment marks which may also resemble M- or W-shapes. The caudal fin usually has 2-4 (up to 6) complete bars. Breeding males are black and flank markings are not always visible or appear as bars. The dorsal, anal and pelvic fin spine tips may be swollen white knobs.
Size
Reaches 7.7 cm.
Found in southern Québec, Ontario, Manitoba and eastern Saskatchewan. Occurs southward to Mississippi and Alabama.
Origin
This species entered the NCR in a northward dispersal from a Mississippi refugium via the Great Lakes and Ohio River drainages, 12,000 years B.P. (Chapleau and Cooper, 1992).
Habitat
The Johnny Darter occurs in lakes, large rivers and streams although preferring the latter. It is found in relatively fast water or in still water and is most frequent over sand, gravel or boulder bottoms. In competition with other darters, it dominates weed beds. It may also be found in lakes down to 64 m. Its preferred temperature is 22.8°C. It is less sensitive to pollution compared to other darters in the NCR.
Age and Growth
Females live about a year longer than males, to age 4, but males are larger. They can mature at 1 year.
Food
Food is various aquatic insects and crustaceans taken from the bottom. This common darter is eaten by many other fishes.
Reproduction
Spawning occurs from April to June in Canada, the peak depending on environmental conditions (temperature range 10-25°). Males clean the undersurface of rocks with their fins and maintain a territory, defending it against other males by a display with erect fins. Male fights involve head butting and fin nipping. Males also defend the nest site against crayfish which favour territories under rocks and eat fish eggs. Crayfish with a carapace length up to 3.2 cm were chased away by Johnny Darter nips to the abdomen. Females are courted by the male's invitation under the rock and his movements there upside down. The pair move over the rock surface together inverted and an adhesive egg is deposited and fertilised every few seconds. Should the female turn upright, the male prods her into inverting and continuing the egg laying. The female may deposit 30-200 eggs at each site and visit 5-6 sites. A single site may have up to 1150 eggs, collected from a series of females. Males guard the eggs and fan them with their pectoral fins to keep them clean and aerated. The eggs are also rubbed with the swollen dorsal fin spines. Eggs which develop a fungal infection are eaten. Eggs are up to 1.5 mm in diameter.
Importance
This species is an excellent aquarium denizen and has been used in behavioural studies.
Tessellated Darter / Raseux-de-terre gris
Etheostoma olmstedi Storer, 1842
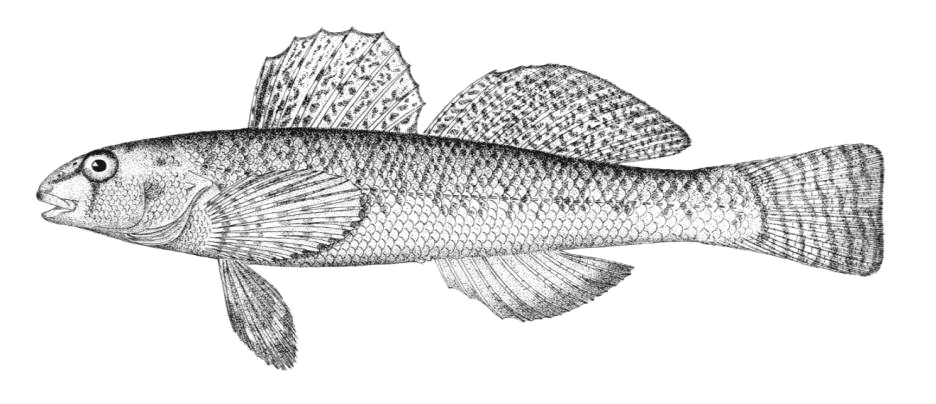
Taxonomy
Also known as dard tesselé. This species was long confused with the Johnny Darter and so comparatively little is known of its biology in Canada. It hybridises with the Johnny Darter in the NCR (McAllister et al., 1972). Some authors consider it to be a subspecies of Etheostoma nigrum (Goodchild, 1994a).
Key Characters
This darter is recognised by the small mouth not extending beyond the anterior eye margin, a smooth edge to the preopercle bone on the side of the head, an anal fin smaller than the soft dorsal fin, belly scaled or naked but no enlarged scales in mid-line, premaxillaries protractile, i.e. a deep groove separates the upper lip from the snout, 1 thin anal spine, 9-13, usually 11, pores in the preoperculomandibular canal (the head sensory canal running from the lower jaw onto the preopercle bone), and 10-17, usually 13 or more, dorsal fin soft rays. Usually confused with the Johnny Darter but has continuous infraorbital (under the eye) and supratemporal (over back of head) canals.
Description
Dorsal fin spines 5-11 (usually 8-10), anal fin usually with 1 spine in Canada and 5-11 (usually 7-9) soft rays, and pectoral fin rays 11-14. Lateral line scales 34-64. There are 7-10 short gill rakers. Scales on the nape, breast, belly and cheek vary from absent to fully scaled but are more scaly than the Johnny Darter.
Colour
The back is yellowish to light green and has 6 dark brown saddles. The flank has 7-12, usually 8-11, brown w-, x-, or v-shaped marks. The belly is white. There is a bar below the eye and in front of the eye. The caudal fin has 4-11, usually 5-8, complete bars and the dorsal and pectoral fins are also barred. There is a faint spot at the caudal fin base. Breeding males develop 12-13 bars along the flank and lose the w-, x-, and v-shaped markings. Fin membranes become dark and the unpigmented rays stand out in contrast, the reverse of the non-breeding appearance. Breeding males have a blotch between the first 2 spines of the dorsal fin. Pelvic spine and ray tips form white knobs. The second dorsal fin is large and often reaches back to the caudal fin.
Size
Reaches 8.8 cm standard length.
Found in Atlantic drainages from southeastern Ontario and southwestern Québec south to Georgia and Florida but not in maritime Canada.
Origin
This species entered the NCR from an Atlantic coastal refugium.
Habitat
This darter prefers larger rivers, but may also occur in streams and lakes, and is generally found over sand, mud or rock bottoms in slow to still water. It is replaced in some streams by the Johnny Darter. It is tolerant of polluted water. The preferred temperature is 22.8°C. They are often buried in the sandy substrate with only the eyes and caudal fin tip showing.
Age and Growth
Maximum age is between 3 and 4 years. All are mature at 2 years, and some at 1 year of age.
Food
Food is crustaceans, insects, snails and algae taken mostly during the day.
Reproduction
Eggs are laid in clusters up to 7.5 cm wide on the underside of rocks in spring (April-June), when both sexes are upside down. Temperatures range from 12.5-18.5°C and spawning usually occurs during the day, although it may occur at night too. Current is moderate and water depth is a few centimetres to over 2 m. The eggs are guarded and cleaned by the male. Up to 1435 eggs are laid with a diameter up to 1.6 mm. They hatch in 97 hours at 25-26°C. Eggs are laid in clutches of 19-324. This darter is unique among fishes in that males clean and defend eggs which they did not fertilise. A nest may contain over 2000 eggs. Large males are dominant over smaller males and defend the few rocks in a stream which can be used for spawning. However the dominant male will move from his original rock to others because they have more uncovered spawning area and attract females. Subordinate males take over the abandoned rock, clean the surface not yet used and also clean the dominant male's eggs. In effect the dominant male is exploiting the subordinate male's lack of access to spawning sites. The dominant male can spawn repeatedly at different sites but is assured of care of "his" eggs by the subordinate male.
Importance
The Committee on the Status of Endangered Wildlife in Canada placed this species in the "Not at Risk" category in 1993.
Yellow Perch / Perchaude
Perca flavescens (Mitchill, 1814)
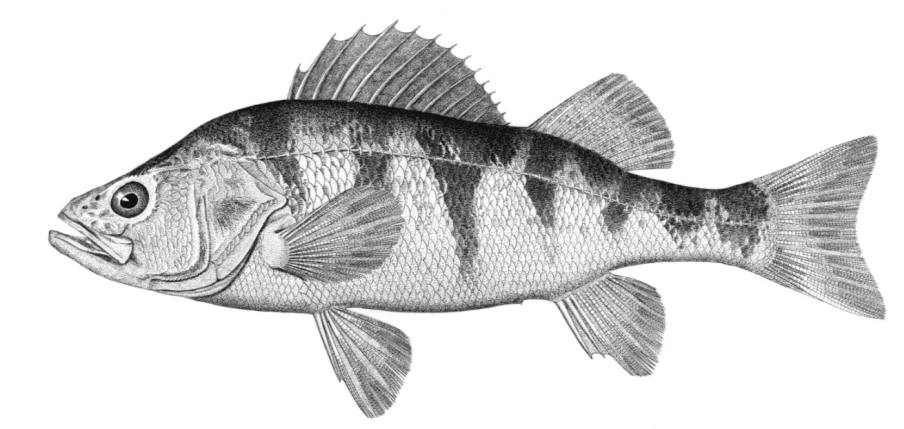

Taxonomy
Other common names include Common Perch, Lake Perch, American Perch, Raccoon Perch, Coontail, Ring Perch, Striped Perch, Redfin Perch, Convict, Yellow Ned and Jack Perch.
Key Characters
The most distinctive feature of the perch is the 5-10 wide black bands on the flank although these may be faint in some populations. In addition the mouth is large with the upper jaw extending back to the middle of the eye or further, the preopercle bone on the side of the head is serrated at its angle, and there are only 6-9, usually 7-8, soft rays in the anal fin.
Description
Lower jaw teeth are small and none are canines. First dorsal fin spines 11-15, second dorsal fin with 1-2 spines and 12-16 soft rays. There are 2 anal fin spines. Pectoral rays 13-15. There are 19-22 moderately slender gill rakers. The space between the pelvic fin bases is less than 1 base width. Ctenoid scales number 50-70 in the lateral line. Cheeks are scaled. There are 3 short, thick, pyloric caeca.
Colour
The back and sides are green, olive or yellow-brown fading ventrally to grey or white. The flanks may be a rich golden yellow. The eye is yellow or green. The spiny dorsal fin is yellowish to green with a black margin and often with black on the membranes anteriorly and posteriorly. Other fins are usually yellowish but the pectoral and pelvic fins may be more orange. The pelvic fins may be silvery. Fin membranes may be clear to cloudy and usually not coloured. Breeding males have orange to bright red lower fins and colours generally are more intense. Faber (1985a) illustrates a larva.
Size
Reaches 53.3 cm. The world, all-tackle angling record from Bordentown, New Jersey in 1865 weighed 1.914 kg. The Ontario record as of the year 2000 weighed 1.0 kg.
Found from Nova Scotia except Cape Breton Island, through New Brunswick, southwest Quebec, Ontario and Manitoba including the Hudson Bay lowlands, Saskatchewan, Alberta and north to Great Slave Lake. Present in southern Columbia River drainages of British Columbia from the spread of specimens introduced to Washington. In the U.S.A. south to Missouri and to South Carolina east of the Appalachians. Widely introduced outside this natural range.
Origin
This species entered the NCR from a Mississippian or an Atlantic coastal refugium and possibly a Missourian refugium (Mandrak and Crossman, 1992). The presence of this species in Gatineau Park may be from introductions (Rubec, 1975a).
Habitat
Perch are commonly found in lakes, ponds and rivers where there is some vegetation, clear open water, slow to no current and low turbidity. They are often found associated with structures in the water, whether natural or man-made like breakwaters and boat docks. Preferred temperatures are in the 20-24°C range (21.4°C) and they are limited by the 31°C summer isotherm in the south. pH as low as 4.4 is tolerated. Depth range is down to 46 m but they are mostly found in water shallower than about 9 m. They travel in schools of up to 200 fish, daily and seasonally for spawning and feeding and in response to temperatures. Schools of young perch may be mixed in with schools of Spottail Shiners swimming near the surface. Predators such as the larger Yellow Perch, Northern Pike and Walleye swim below the mixed school and are 10 times more likely to take a shiner than a perch. They rest at night on the lake or river bottom and, unlike Walleyes and Saugers, are most active during the day. Schooling by adult, predatory perch reduces the chances of a prey fish to escape - it may encounter another school member. Yellow Perch are active under winter ice.
A study on perch in the Ottawa River (Stobo, 1971) showed 65-100% mortality below effluents for caged fish and mortalities of 10-20% up to a mile below, partly due to less food and less variable food supplies than in natural areas. Tag recoveries at 56.5% indicated a restricted home range and populations in the river are possibly distinct.
A fish kill along extensive stretches of the Rideau River and Canal was reported in spring 1956 (newspaper reports). The raising of water levels in the Rideau River and Canal system starting in late April 1996 was detrimental to perch populations. The lowering of the sluice gates at Hog's Back, to fill canal sections to the south, cut off water flow to the north. Submerged aquatic vegetation near Carleton University was exposed stranding both eggs and adult fish (RMOC, 1996a).
Age and Growth
Young-of-the-year perch in the Ottawa River show linear growth from 73 to 114 mm fork length from 3 June to 26 September. Growth of these fish over winter was negligible as fish caught the following May were of similar size to those caught in late September (Rodgers, 1976). Females grow faster than males and reach a larger final size. Males are usually mature at 2-3 years at females at 2-4 years but this varies with latitude as does growth. Some one-year-old males are mature in Lake Erie. In Québec it takes 4-5 years minimum to produce fish of a harvestable size (18-20 cm). Maximum age may be about 21 years although 13 years is a more usual figure in the literature. In the Ottawa River at Ottawa 5 age groups are reported (Qadri and Rubec, 1974) while Pluritec Ltée (1982) record a 11+ fish from Lac Philippe, Gatineau Park. In the Plantagenet Reach of the South Nation River an age range of 2-6 years was recorded (Lauzon, 2003) and fish up to 11 years are reported from the Rideau River (Setterington, 2004). Stobo (1971) showed that fish from polluted areas in the Ottawa River had decreased growth compared to more pristine areas in the NCR. Maximum age was 7 years for both sexes.
Perch in rivers of James Bay have a slower growth rate and shorter maximum length but longer life span than perch from southern Québec. Some populations of perch comprise stunted fish because of their strong appetite, high rate of reproduction, competition for food and such factors as predators and available space. Stunting is often a feature of small lakes while the best growth occurs in large water systems. A stunted population has been known from Lac Hertel, Québec for over 20 years yet when young perch were grown under optimal conditions in the laboratory, their growth was comparable to normal populations. Lac du Printemps in Gatineau Park contains only perch and this population has been stunted for at least a decade (Ridgway and Chapleau, 1994). Females grew faster than males and reached greater lengths and ages (24.1 cm and 10 years compared to 17.2 cm and 7 years). Growth rate was well below the average for other Québec populations of perch, age-classes converged towards a similar size at earlier ages and males and females reached maturity at earlier ages (0+ and 1+ respectively), all features consistent with stunting. The average gonadosomatic index of 0+ males was 9.3%, one of the highest ever reported for perch. Perch biomass in small lakes and ponds without other species can reach 215 kg/ha but in lakes with other species it is usually under 65 kg/ha.
Food
Food changes with age but is principally insects and other invertebrates, and fishes including all life stages. Young-of-the-year perch feed diurnally and have a diet dominated by benthic invertebrates (amphipods, chironomids, ephemeropterans, trichopterans and plecopterans) through the summer in the Ottawa River with zooplankton (cladocerans, copepods) important in June and fish (Hybognathus regius) in August and September. These changes reflect the availability of prey (Rodgers, 1976; Rodgers and Qadri, 1982). Peak feeding occurs at sunrise and sunset. Perch may hunt in packs which improves the chance of capturing active prey. Large perch eat young perch. Older perch in Lac du Printemps, Gatineau Park did not eat young-of-the-year in October because of their large size and this may contribute to stunting. Perch are food for many other fishes and birds but also compete with such sport fishes as trout and bass. Perch are a major food of Walleye and are eaten by Northern Pike and Smallmouth Bass in Lac LaPêche, Gatineau Park and by Smallmouth Bass in Lac Meach (Pluritec Ltée, 1982).
Reproduction
Spawning occurs usually from mid-April to May but may be as late as July. Egg masses have been seen attached to bottom debris in the Rideau Canal in downtown Ottawa (Wachelka et al., 2000). A spawning run of perch occurs in the Rideau River near Carleton University below the train bridge in early May (Hopkins, 2000). There is a spawning migration upriver or onto lake shallows and spawning occurs at temperatures of 5.6-19.0°C. Fish return to the same spawning ground in subsequent years. Males arrive earlier and stay longer than females on the spawning grounds. Spawning takes place during the night and early morning hours over vegetation, roots or fallen trees or over sand and gravel. Each female is attended by a double line of males, numbering up to 25! The first 2 males keep their noses pushing the female's belly and the remaining males follow as the female pursues a curved course. The female lays up to 210,000 eggs of 3.5 mm diameter in a twisted, gelatinous, transparent string up to 2.1 m long and 10.2 cm wide, weighing as much as 2 kg. The string has a central tube and holes to afford oxygen circulation and is folded in convolutions like an accordion. The egg mass adheres to vegetation and is often twisted around it during a spiral clockwise movement by the female.
Importance
Yellow Perch are important commercially, as sport fish and are used in schools and universities to typify a fish for anatomy classes. In the Ottawa River it has been used to develop a pollutant accumulation model which predicts uptake of PCBs and methylmercury in fish tissues (Norstrom et al., 1976). Anglers take this species in both summer and winter and enjoy its white, flaky flesh. Bait is usually minnows, worms or cut fish and perch can be caught on plugs, spoons and even flies. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Commercial fisheries centre on the Great Lakes where pollution and overfishing have taken their toll. A catch of 32.7 million kg was made in the Great Lakes in 1934 but catches have been as low as 4% of this figure. The total Canadian catch in 1988 was 6400 tonnes. Dymond (1939) records catches from 1896 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 42,000 lbs (19,068 kg) in the Ottawa River from Carillon to Pontiac (Québec), the highest recorded. On the Ontario side the catch was highest in 1896 at 9400 lbs (4268 kg) from Prescott and Carleton counties. Commercial fisheries for perch above and below Hull from the Québec side of the Ottawa River is documented by Pluritec (1982b) for the period before 1964 and for the Ontario side of the Ottawa River from Carillon to Ottawa-Hull (Ontario Ministry of Natural Resources (OMNR) and Gouvernement du Québec Faune et Parcs,1999). Unbreaded perch fillets sold for as much at $17.50/kg in the mid-1980's and demand exceeded supply. In August 2005 in St. Jacobs, southern Ontario, perch sold for $12.99 a pound (lb).
Logperch / Fouille-roche zébré
Percina caprodes (Rafinesque, 1818)
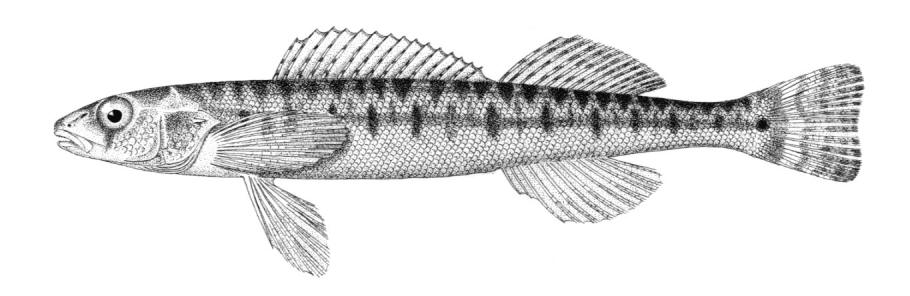

Taxonomy
Other common names include Zebra Fish, Jackfish, Manitou Darter, Rockfish, Hogmolly, Hogfish, Fouille-roche zébré and Dard-perche. Percina caprodes semifasciata (DeKay, 1842), the Northern Logperch, is the subspecies in the NCR.
Key Characters
This darter is separated from its relatives by having an obviously protruding snout, a large anal fin at least as large as the soft dorsal fin, belly mid-line either naked (females) or with 20-37 enlarged scales (males), 2 anal fin spines, no groove between the snout and lip (premaxillaries not protractile), 67-103 lateral line scales and, most distinctively, by 14-25 flank bars.
Description
First dorsal fin spines 12-17, usually 14-16, second dorsal rays 13-18, usually 15-16, soft anal fin rays 8-13, usually 10-11. Pectoral fin rays 12-16, usually 14-15. Short gill rakers number 14-20. The cheeks and opercles are scaled and the breast is naked. This darter is very variable in characters over its wide range.
Colour
Overall colour is yellow-green to grey-green. The back has 8-10 saddles. The flank bars may terminate in a ventral tear-drop. Alternate bars reach to, and pass below, the lateral line. The dorsal fins are striped orange and black and the caudal fin has 3-4 bars. The caudal fin has a large black basal spot. The pectoral and pelvic fins are mostly clear but may have yellow tinges. The ventral scales develop tubercles in spawning males, which are darker than females but have no bright colours except sometimes for orange around the caudal spot.
Size
Reaches 17.8 cm, perhaps 20.0 cm.
Found from east-central Alberta, central Saskatchewan, Manitoba, Ontario and southeastern Québec south to the Gulf of Mexico. Also reported from the north shore of the Gulf of St. Lawrence opposite Anticosti Island as an isolated population.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
This species has the widest distribution of any darter and is found in streams, rivers and lakes over a variety of bottoms and in varying currents. It may even be found buried in sand with only the eyes showing. It tends to prefer rocky substrates with little vegetation. Reported down to 40 m in Lake Erie but can be commonly found in shallows. Spawning takes place at temperatures over 16°C in Manitoba.
Age and Growth
Maximum age is about 4 years with maturity at 2 years.
Food
Food includes aquatic insects and crustaceans and some molluscs revealed when stones and debris are overturned by the snout. Logperch also eat fish eggs and non-spawning males are fond of eggs of their more successful siblings.
Reproduction
Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 6 May to 25 May at 10-14ºC. Spawning occurs from early May to early August generally when males move into sandy shallows of lakes in schools of up to several hundred fish or into streams with shallow rapids. Females swim through this school, and if one stops on the sand, a male will settle on her back, clasping with his pelvic fins and depressing his caudal fin alongside hers. Both fish quiver and eggs are fertilised in a cloud of sand which coats the eggs. The eggs are not guarded and males do not defend a territory. In streams males have a moving territory around a female as she swims onto the spawning bed. Both lake and stream spawning behaviours of Logperch are very primitive among darters. About 10-20 colourless eggs are released each time and the female may spawn with several males. Eggs are 1.3 mm in diameter and number up to 3085 in large females. Young Logperch are poorly developed in contrast to other darters and must drift from flowing to still waters where suitable small plankton is available as prey. Other darters can start feeding immediately on small insects.
Importance
Logperch thrive in an aquarium and are used as bait fish for Smallmouth Bass among others in Canada.
Channel Darter / Fouille-roche gris
Percina copelandi (Jordan, 1877)
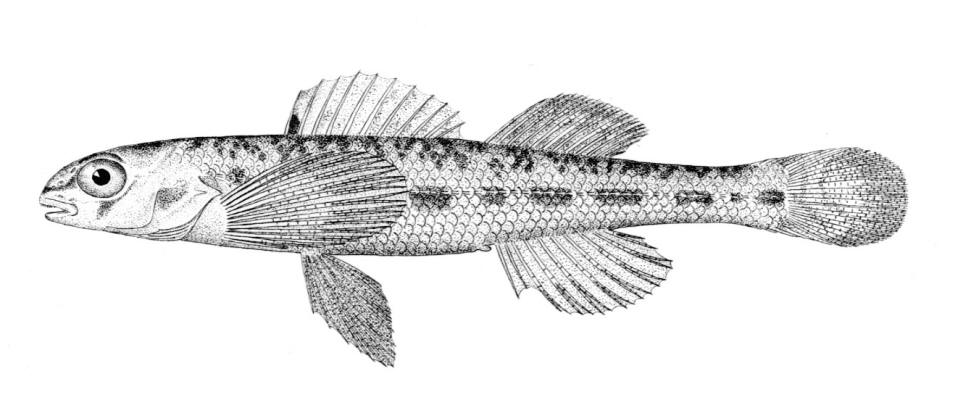
Taxonomy
Other common names are Copeland's Darter or Dard gris. It hybridises with the Logperch.
Key Characters
A small member of the family, this darter species is recognised by the small mouth not extending beyond the anterior eye margin, a smooth edge to the preopercle bone on the side of the head, a large anal fin at least as large as the soft dorsal fin, belly midline either naked or with enlarged scales, 2 anal fin spines and a deep groove between the snout and lip (premaxillaries protractile).
Description
Dorsal fin spines 9-13 (usually 10-11), dorsal soft rays 10-14 (usually 11-12), anal fin rays 7-10 (usually 8-9). Lateral scale rows 43-61. Males have 6-11 enlarged, modified, star-shaped scales in a row on the belly where females are unscaled or scaled only posteriorly. Tubercles are present. Females have an elongate tubular papilla in the genital area.
Colour
Overall colour is brown to olive, semi-translucent, with about 5-9 faint saddles. The flank has 8-18 brown to black oblong and partly confluent blotches linked by a faint, thin brown to black stripe. A dusky bar or spot may extend from beneath the eye to the snout. Fins are mostly clear. There is usually a spot at the caudal fin base and 2 bars radiating down and forward from the eye. Breeding males have black basal and marginal bands with white at the tip on the spiny dorsal fin, a black head, throat and pelvic fins, a greenish cast to the upper flank and a bluish one to the lower flank. The basal part of the anal fin is blackish.
Size
Reaches 6.1 cm standard length or 7.3 cm total length.
Found in the upper St. Lawrence River basin east and west of Montréal and at Ottawa, and along the shores of lakes Erie and Huron. It is only found west of the Appalachians in the U.S.A., south to the Gulf of Mexico. Boucher et al. (2009) collected it from the Gatineau River in a 6 km section above the confluence with the Ottawa River.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
A generally uncommon darter found over sand, gravel and rock mixes in larger rivers with moderate current, stream pools, and on lake shores where current is slow and depths are shallow. In the Gatineau River it is recorded from lotic zones where periphyton is present and there is a mean current velocity of 41 cm.s-1 (Boucher et al., 2009). It may move into very shallow waters at night. It is intolerant of pollution. Competition with other darters, such as Etheostoma nigrum and Percina caprodes, for spawning territory may limit this species.
Age and Growth
Males grow larger than females. Maximum age is 5 years with maturity at 1-2 years.
Food
Insects and crustaceans are the major foods, and large amounts of algae and detritus are also swallowed.
Reproduction
Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 20 May to 21 June at 14-19ºC. Spawning occurs generally in relatively shallow running water in June in Canada at 14.5-25.0°C, July in the northern U.S.A. at temperatures over 20°C, perhaps earlier (May-June) in other localities. A short migration is made to appropriate spawning sites. Males have a territory based on a central rock. Females join the male over gravel or small rocks behind the central rock. The female partly buries herself in the gravel, the male presses down on her from above with his pelvic fins on each side and his tail area paralleling hers, and adhesive eggs are released and fertilised into the gravel. Several females may spawn with 1 male, depositing 4-10 eggs. Each female may have over 720 eggs up to 1.4 mm in diameter. The eggs are not guarded by the parents. The Farmers rapids on the Gatineau River are a spawning site (www.fapaq.gouv.qc.ca, downloaded 7 October 2002).
Importance
This darter was given the status "threatened" in 2002 by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2002). Boucher et al. (2009) consider that this species is doing well in the Gatineau River.
Sauger / Doré noir
Sander canadensis (Griffith and Smith, 1834)
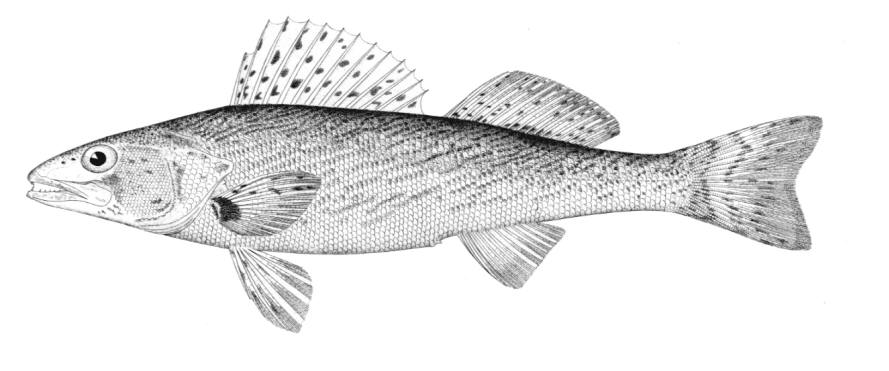

Taxonomy
Other common names include Sand, Blue, or Grey Pickerel, Pike or Pike-perch, River Pike, Spotfin Pike, Pickering, Jack Salmon, Horsefish, Spotted Trout and Rattlesnake Pike. Saugeyes are hybrids between Sauger and Walleye, characterised by partially scaled cheeks, a yellower colour than either parental species but with rows of spots like the Sauger, and more than 3 pyloric caeca (as in Sauger) but these are as long as the stomach (as in Walleye).
Key Characters
This species has a large mouth with the upper jaw extending back to the rear edge of the pupil, the preopercle bone on the side of the head is serrated at its outer angle, there are 10-14 soft rays in the anal fin after 2 spines, 2 canine teeth are present at the lower jaw tip, the spiny dorsal fin is clear with small spots and there are 3-4 brown saddles on the back.
Description
The Sauger is more slender than the Walleye and has a pointed snout. First dorsal fin spines 10-15, second dorsal fin with 1-2 spines and 16-22 soft rays. Pectoral fin rays 12-14. There are 3-9, usually 5, pyloric caeca attached to, but shorter than, the stomach. Gill rakers 6-8 on the lower limb and 3-5 on the upper limb, moderately long and denticulate. Scales number 78-100 in the lateral line.
Colour
Overall colour yellowish-brown, golden olive, or even grey, saddles are a darker brown. Flanks may have several large, dark brown spots. Generally this species is darker than the Walleye taken from the same waters. Belly a milky-white. The first dorsal fin has a dark margin and the membranes are spotted black in 2-3 rows, each spot being a half-moon shape. The second dorsal fin has 2 rows of spots forming narrow bands. The caudal fin is also barred and its lower margin may be white. The anal and pelvic fins are clearer but have dark specks. The pectoral fin has a black, basal spot.
Size
Reaches 76.2 cm. The world, all-tackle angling record weighed 3.96 kg and was caught in 1971 in Lake Sakakawea, North Dakota. A 3.52 kg Sauger from the Saskatchewan River, Saskatchewan is the Canadian record. The largest Sauger may be Saugeyes, hybrids with Walleye.
Found from southern Canada southward west of the Appalachians to Arkansas and Tennessee but introduced into some eastern and southern areas. In Canada from the North Saskatchewan River in Alberta and east to James Bay drainages, essentially in the southern half of Alberta, Saskatchewan and Manitoba, to southwestern Quebec and waters southward.
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
Saugers prefer more turbid water than Walleyes whether in large, shallow lakes or large rivers with slow current. They are widely distributed in the Ottawa River in the NCR but absent from most local lakes. Sauger are abundant in new reservoirs which tend to be turbid but their numbers decrease as the reservoir stabilises. Turbid conditions may protect young fish from predators by reducing visibility, prevent eggs from sticking together and being deprived of adequate oxygen supplies and may concentrate plankton to lighted surface waters where the young sauger can feed easily. Sauger are usually found in the upper part of the water column but may descend to 20 m. The tapetum lucidum, a layer in the eye which causes a silvery eyeshine, serves to stimulate the visual cells by reflection. In low light conditions of turbid waters, vision is enhanced and since the Sauger's tapetum is better developed than that of a Walleye's, it is at an advantage in turbid water. Sauger are more active in shallow water when it is windy, and presumably waves are increasing turbidity. Its preferred temperature is 19.2°C. In the Ottawa River, eutrophication from impoundments and other human activities mean that this species and it's relative, the Walleye, compete for the same prey in the littoral zone. Prey species are excluded from the sublittoral zone because of warmer temperatures and siltation. As a result, Osterberg (1978) predicted that Sauger will disappear from the Ottawa River. Two-thirds of Sauger moved into feeding areas in Governor's Bay in the Ottawa River between sunset and midnight, most of the rest before 3 a.m. They leave the bay in daylight hours to be in 5-13 m depths around rocks and debris (Osterberg, 1978).
Age and Growth
Growth varies with the habitat, richer waters showing more rapid growth than poor waters such as those on the Precambrian Shield. Old, slow-growing populations in the north reach 18 years while young, fast-growing populations in the south are up to 6 years old. Sauger in rivers draining to James Bay have a slower growth rate, shorter maximum length but a longer life span than Sauger in southern Québec. Males are mature at 2 years and females at 3-4 years of age. Sauger in the Ottawa River reach 9 years (McAllister and Coad, 1975). Osterberg (1978) showed that growth in the Ottawa River was the lowest recorded for this latitude, the mean total length at 6 years being 29.4 cm while in the Great Lakes it was 41 cm. Growth is less rapid than in the related Walleye. At age 5 the average Walleye in the Ottawa River was 37 cm long and weighed 481 g and life span was 8 years in Osterberg's study. Growth was faster below Ottawa than above, 50% of 3-year-old males were mature and 50% of 4-year-old females.
Food
Food of small Sauger is plankton, fish fry and insect larvae, with larger Sauger taking crayfish, insects and a variety of other fishes. Sauger are known to favour demersal fish prey throughout the day in some habitats and can be cannibals when other prey is less abundant. In Governor's Bay on the Ottawa River, the Emerald Shiner Notropis atherinoides, is the main food item at more than 75%, taken in nocturnal feeding movements into the bay, with Yellow Perch being an important secondary food when shiners were at a low density (Osterberg, 1978; Qadri and Rubec, 1974). Osterberg (1978) found that feeding peaked in August and food passed through the gut in 48 hours. Yellow Perch are predators of Sauger in the Ottawa River (Osterberg, 1978).
Reproduction
Spawning occurs at the end of May and beginning of June on gravel shoals down to 6.1 m of rivers or lakes at about 4-11°C. Males arrive first on the spawning ground while females are the first to leave. Spawning takes place at night with each female accompanied by one to several males. The eggs are shed, fertilised and fall into interstices of the gravel. A large female may produce up to about 210,000, non-adhesive eggs of 1.5 mm diameter.
Importance
Sauger are important commercially. They are caught with gill nets, pound nets and trap nets and in Manitoba as much as 2.5 million kg may be caught annually. The catch in Lake Erie has declined from 2.7 million kg to almost nothing. Anglers may catch Sauger on minnow or frog baited hooks, trolled or drifted, or with various artificial lures and it is a strong fighter although not as popular a sport fish as Walleye. Sauger are good eating with a firm white flesh sold usually as fillets. Few anglers pursue this species in the NCR, preferring Walleye. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
Walleye / Doré jaune
Sander vitreus (Mitchill, 1818)
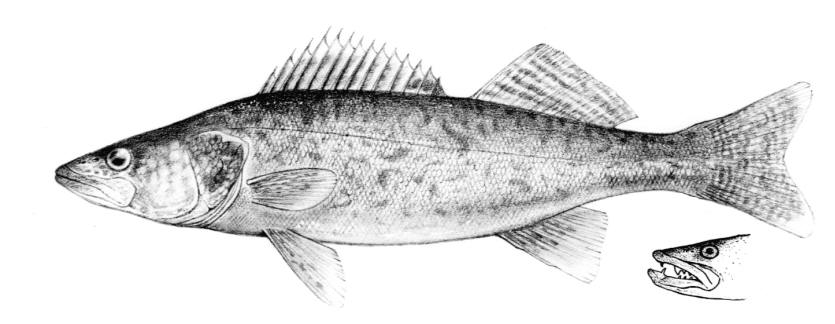



Taxonomy
Other common names include various combinations of Yellow, Walleye, Pickerel and Pike; Pike-perch, Dory, Gray Pike, Green Pike, Jack Salmon, Glass Eye, Marble Eye, Doré, Doré blanc and Doré bleu. Anglers often call the Walleye "pickerel" but the words pickerel and pike should be confined to members of the unrelated Pike Family. Hybrids with Sauger are known (see above).
Key Characters
The Walleye has a large mouth with the upper jaw extending back to the rear edge of the eye, the preopercle bone on the side of the head is serrated at its outer angle, there are 10-14 soft rays in the anal fin after 2-3 spines, 2 canine teeth are present at the lower jaw tip, in particular there is a large, black blotch at the rear base of the spiny dorsal fin, and 5-8 saddles on the back.
Description
First dorsal fin spines 11-16, second dorsal fin with 1-2 spines and 17-22 soft rays, and pectoral rays 13-16. Lateral line scales 77-108. Gill rakers 11-17. There are 3 pyloric caeca attached to, and as long as, the stomach.
Colour
Overall colour has brassy tones and is dark green, olive-brown or yellow with gold flecks on the sides. The belly is white to yellowish. The lower lobe tip in the caudal fin and the anal fin tip are white. Males have a more pronounced white lower lobe to the caudal fin. The spiny dorsal fin membranes are not spotted but dusky. The second dorsal and caudal fins are speckled in rows. Pelvic fins are yellowish to orangish. Pectoral fins have a dark, basal blotch. The peritoneum is white. The eyes are silvery from reflections of the tapetum lucidum and the cornea is milky (hence "walleye"). Young fish often have 4-14 bars on the flank, particularly in clear water. Faber (1985a) illustrates a larva.
Size
Reaches 1.07 m and 11.42 kg. The world, all-tackle angling record weighed 11.34 kg and was caught in 1960 in Old Hickory Lake, Tennessee. The Ontario record as of the year 2000 weighed 10.1 kg. Fish up to 8.6 kg are known from the Lièvre River (Campeau, 2002), and a 10 lbs 2 oz fish from the Lièvre was only 28 inches long but 18 inches in girth (http://iquebec.ifrance.com/maclaren/johnsim1.htm). Fish in the 7-9 lb range are caught at Petrie Island in the Ottawa River (Ottawa Sun, 10 June 2004), a 28 inch fish was caught there in mid-May 1996 (www.fish-hawk.net/gallery/walleye/Walleye-jun01.htm), another on New Year's day was more than 12 pounds (Hopkins, 1995), and one from there, caught through an 8-inch hole in the ice by Bob Presland on 12 January 2004, was 15 lbs 4 oz and 32 inches long (newspaper reports). A 10 lb 5 oz fish was taken near the Connaught Ranges (newspaper report); a 9 lb fish was taken at the Champlain Bridge on the Ottawa River (Anonymous, 1962b); a 27 inches and 7 lbs fish from the Mississippi River on 17 July 1999 (www.fish-hawk.net/gallery/walleye/Walleye-jun01.htm); a 9 lbs fish from the Gatineau River on 15 September 1999 (www.fish-hawk.net/gallery/walleye/Walleye-jun01.htm); a 9 lbs and 28.5 inch fish was taken on the Rideau River on 4 January 2003 (www.fishontario,com, downloaded 20 May 2003) and also a 14.5 lbs fish from the Rideau River (www.rideaufriends.com/fishing.html, downloaded 20 June 2003); 8lbs 4 oz in Dow's Lake on 24 December 1984 (www.ottawafishing.com, downloaded 20 May 2003); a nearly 10 lbs fish at Wendover on the Ottawa River (www.fish-hawk.net, downloaded 20 May 2003); a 10 lbs 9.6 oz and 28 inch long fish from Fitzroy Harbour on 17 January 2004 (www.fishontario.com/photo_gallery/page2/page2.html, downloaded 23 June 2004).
Found from western Quebec, throughout Ontario, Manitoba, Saskatchewan and Alberta and north to the mouth of the Mackenzie River including Great Bear and Great Slave lakes and extreme northeastern British Columbia, and southward to the Gulf of Mexico in Alabama. Also introduced outside native range. This species has been stocked in Mississippi Lake in 1908 (Brown, 1984), in the South Nation River system, 5.5 million fry being released prior to 1950 (Anonymous, 1950). Lac LaPêche was stocked with about 4.5 million fry in 1968 but this may have failed due to predatory Yellow Perch while 200 adults stocked in the same lake appear not to have reproduced (Rubec, 1972). The Ottawa and Rideau rivers were heavily stocked in the 1940s and early 1950s and again in the 1980s. One million eggs were planted between Kars and Beckett's Landing in 1942 and 200,000 eggs were planted near Kars in 1947 (Bebee, 2004). The 1980s stocking improved fishing between Burritts Rapids and Kars for a short period (Hopkins, 2000). The Ottawa River stocking in 1947 comprised 200,000 eyed eggs released at Woodruffe below the Britannia Rapids, 200,000 at Galetta, 200,000 in the Madawaska and Ottawa rivers near Arnprior and 100,000 at Rockland (newspaper reports). Other reports form this period list releases of a million eggs in both the Rideau and Ottawa rivers.
Origin
This species entered the NCR from a Mississippian or Atlantic coastal refugium and possibly from a Beringian or Missourian refugium (Mandrak and Crossman, 1992). The presence of this species in Lac Philippe, Gatineau Park is an unauthorised introductions (Chapleau et al., 1997).
Habitat
Walleye favour both turbid and clear lakes but the very sensitive eyes limits their activity in clear water. They rest on the bottom during the day in clear waters. They prefer less turbid conditions and shallower water with slower current than Sauger. Large streams and rivers which are turbid or have deep, sheltered pools, weed beds or fallen trees, also harbour Walleye. A temperature range of 0-30°C is tolerated although 20-23°C is preferred. A fish kill along extensive stretches of the Rideau River and Canal was reported in spring 1956 (newspaper reports). They are active in winter and swim in schools often associated with other sport fish such as Northern Pike, Yellow Perch, Muskellunge and Smallmouth Bass. Water depth preferred is moderate, down to about 21 m, usually shallower. There are morning and evening migrations into shallow or surface waters to feed. Some fish have been recorded as moving 160 km, but this movement is unusual apart from regular spawning migrations. Walleye have been tagged with radio transmitters in the Jock River of the NCR to monitor their movement as they are an indicator species for good habitats (Spears, 1999a). Tagged Walleye moved 15 km downriver from Governor's Bay in the Ottawa River (Osterberg, 1978). Two-thirds of Walleye moved into feeding areas in Governor's Bay in the Ottawa River between sunset and midnight, most of the rest before 3 a.m. They leave the bay in daylight hours to be in 5-13 m depths around rocks and debris (Osterberg, 1978).
Age and Growth
Growth varies with latitude and northern populations may be only half the length of southern ones at the end of their first growing season. Northern populations may live 26 years and southern ones up to 12 years. Age groups up to 12 years are recorded for the Ottawa River at Ottawa (Qadri and Rubec, 1974), 17 years for the Mississippi Lake and Mississippi River at Carleton Place (Kerr, 1999c), and 21 years for the Madawaska River at Arnprior based on a von Bertalanffy growth curve (Haxton, 2000). The Madawaska population had an exploitation rate in 1997 of 2% and an annual mortality of 80.6% based on tagged fish recaptures and an annual mortality based on age-frequency distributions of18.4-43.8% for 1997-1999 (within the common mortality range of 40-55% for Walleye generally). Males are usually mature at 2-4 years and about 28 cm while females are usually mature at 3-6 years and about 36 cm or more. However maturity of females is governed by temperature - in the southern U.S.A. it is attained at 2 years but only at 10 years in the N.W.T. Females grow more rapidly than males. Osterberg (1978) showed that growth in the Ottawa River was the lowest recorded for this latitude, the mean total length at 6 years being 40.2 cm while in the Great Lakes it was 49 cm. At age 5 the average Sauger in the Ottawa River was 29 cm long and weighed 186 g and life span was 8 years in Osterberg's study. Growth was faster below Ottawa than above, 50% of 3-year-old males were mature and 50% of 4-year-old females.
Food
Food when adult is a wide variety of available fishes but young and adults can be cannibals if other food is not available. Frogs, mudpuppies and small mammals may also be taken. In the Ottawa River food is mainly Yellow Perch and Emerald Shiners (McAllister and Coad, 1975). In Governor's Bay on the Ottawa River, the Emerald Shiner Notropis atherinoides, is the main food item at more than 75%, taken in nocturnal feeding movements into the bay, with Yellow Perch being an important secondary food when shiners were at a low density (Osterberg, 1978; Qadri and Rubec, 1974). Osterberg (1978) found that feeding peaked in late July and food passed through the gut in 48 hours. Young Walleye are of the same size in a school and attempts at cannibalism may have peculiar results. A chain of up to 4 Walleye may happen when successive fish try to ingest the tail of the one ahead. The end of the chain will eventually digest the tail in its mouth and reject the rest. Young feed on invertebrates and even adults will take mostly mayflies and chironomids when these are emerging. Northern Pike are generally the most important Walleye predator but Yellow Perch are important predators of Walleye in the Ottawa River (Osterberg, 1978).
Reproduction
Spawning occurs in early April in southern Ontario to June or later in the north usually after ice break up at 2.2-15.6°C, usually 5-10°C. Some populations spawn under the ice in lakes. Males arrive on the rocky or gravel spawning ground first. Spawning takes place at night in groups of up to 2 females and 6 males. There is some spawning behaviour with chases, pushing and fin erection before the group swims rapidly into shallow water where the females roll on their sides and release their eggs at the surface. Eggs number up to 615,000, are up to 2.1 mm in diameter and fall into crevices between rocks. Eggs hatch after 12-18 days. Larvae hatch at 7.0-8.0 mm and live in open waters of lakes and rivers. Just south of the NCR there is a "pickerel" run at Innisville on the Mississippi River that is regularly reported in local newspapers. It takes place in the last half of April and early May, lasting up to 3 weeks (Anonymous, 1965; 1966; 1967; Fisher, 1987). The peak of the run in 1965 was 29 April for example. The run is said to have started in 1910 when two game wardens first placed 38 adult pickerel in the rapids (Ottawa Citizen, 29 April 1965). Spawning below the weir at Arnprior in the Madawaska River was completed by 13 May in 1997 and some females were ripe on 27 April in 1999 (Haxton, 2000). There are also runs at Burritts Rapids, Black Rapids and Kilmarnock on the Rideau River (Sarsfield, 1967; Hopkins, 2000), Walleye spawn in the Ottawa River at the base of Rideau Falls in April (Hopkins, 2000), and at the base of Chaudière Falls from mid-April to early May beginning at sunset in water temperatures of 4-8ºC with about 200 fish in 5ºC water at the peak in the last week of April (Osterberg, 1978), among many other sites in the NCR. The Kemptville Creek estuary wetland is a regionally significant spawning site (Schueler et al., 1996). There is an annual contest between poachers and conservation officers at these runs (Fisher, 1987).
Importance
The Walleye is the most economically important fish in Canadian freshwaters. It is both a commercial and sport fish with firm white or pinkish flesh. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the Mississippi Lake and River, Rideau River, South Nation River and Ottawa River. Environnement Québec also has recommended limits, in meals per month (1 to 8) for size of fish (small, medium or large), for such areas as Lac Deschênes at Aylmer and Quyon, Deschênes Rapids, the Ottawa River below Gatineau, above Hull, and at Masson, the Lièvre River above and below Buckingham and the Gatineau River at Chelsea, among others (www.menv.gouv.qc.ca, downloaded 13 October 2004). As these limits are apt to change, anglers consuming this fish should consult the most recent version.
In 1999 it was the second most frequent species at 16.0% (after "basses" at 37.4% of events) sought at competitive fishing events in Ontario, although the proportion of events targeting Walleye had declined substantially from the previous year (Kerr, 1999d). Anglers catch it in both summer and winter using live minnows or artificial lures and drifting and trolling techniques. The best fishing is at dusk or dawn, during the night or on cloudy days. Ontario competitive fishing events in 1999 for Walleye featured minimum size limits (e.g. 12") for tournament entry, functioning live wells were mandatory, "let it live" awards were offered and there were penalties for sick or dead fish (Kerr, 1999d). The peak commercial catch in 1956 weighed 9.5 million kg and was worth $3.1 million. Pollution and overfishing, particularly in Lake Erie, has greatly reduced this resource. Mercury contamination of Walleyes in Lake Erie was discovered in 1970 and led to closure of commercial fisheries. The stock has recovered dramatically indicating overfishing was the main cause of stock decline. The total Canadian catch was an estimated 8153 tonnes in 1988 and was worth $28 million. Dymond (1939) records catches from 1881 onward in the general vicinity of the NCR but trends cannot be determined as fisheries data is recorded from different areas at different dates. For example, in 1898 the catch was 54,750 lbs (24,857 kg) in the Ottawa River from Carillon to Pontiac (Québec), the highest recorded. On the Ontario side the highest catch was in 1896 at 7700 lbs (3496 kg) from Prescott and Carleton counties. Walleye are a favourite of local anglers in the NCR but most are 2-3 lbs (0.9-1.4 kg) (McAllister and Coad, 1975).
Sciaenidae - Drums and Croakers - Tambours
Drums or croakers are found in the Atlantic, Indian and Pacific oceans and some species are permanent residents of freshwater. There are about 270 species with 3 species on Canada's Pacific coast, 2 on the Atlantic coast, and 1 in freshwater Arctic and Atlantic drainages.
These fishes have long dorsal fins with a short section having 6-16 spines separated by a deep notch or gap from 1 spine and 20-35 soft rays. The anal fin has only 1-2 spines and 6-13 soft rays. The tail fin is deeply forked. The mouth is usually on the lower side of a rounded head. Jaw teeth are small but some species have massive throat teeth for crushing molluscs and crustaceans. The lateral line scales run to the tip of the caudal fin. Scales are cycloid or ctenoid and cover the head and body. The chin may have small barbels or conspicuous pores and slits. The head has large canals as part of the lateral line system. The upper edge of the opercle bone is forked, with a bony flap over the gill cover. The swimbladder is complex often with many twig-like or finger-like branches; some have been likened to a carrot, others are hammer or anchor-shaped. The sagittal otolith is very large in many species.
Drums are common shallow water species in warmer areas of the world around estuaries, in bays and on banks. Some are found as deep as 600 m. Freshwater species are common in South America but there is only one such species in North America. The swim bladder is used as a "drum" or resonating chamber to produce sound by the action of the drumming muscles. The sounds include quacks, tapping, grunting and snoring as well as drumming and croaking. These noises were understandably a source of confusion to the first sonar operators. Sounds made by Japanese submarines before the Pearl Harbour attack were thought to be drums. The sounds are used in courtship by males. Some species without swimbladders grind their throat teeth to make sounds. The large sagittal otolith or earstone of one of the inner ear canals has been used as jewelry and a good luck charm. Scales are also used to make jewelry. Some drums are used in aquarium displays. They are popular sport fish and are excellent food fish.
Freshwater Drum / Malachigan
Aplodinotus grunniens Rafinesque, 1819
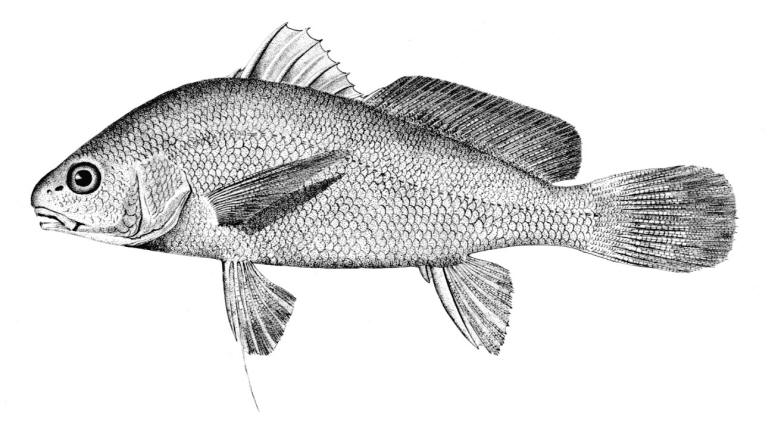


Taxonomy
Other common names include Sheepshead, Silver Bass, Grunter, Red River Bass, Thunder-pumper, White Perch, Goo, Gray Perch, Lake Drum, River Drum, Jewel-head, Bubbler, Grinder, Crocus, Achigan blanc and Gaspergou. "Gaspergou" or casburgot from "casser" (to break) and "burgeau" (a kind of shellfish) is a Cajun word.
Key Characters
This is the only freshwater species of this family in Canada. The massive second anal fin spine and lateral line running to the end of the tail are diagnostic.
Description
The first dorsal fin has 8-10 spines, the second dorsal fin with 1 spine and 24-33 soft rays. The anal fin has 2 spines and 7-8 branched rays. The lateral line to the tail base has 48-53 scales. Short gill rakers number 19-21.
Colour
The back is dark green to olive-brown, bronze or silvery-bluish. Flanks are silvery to bluish or purplish and the belly is white. Fins are dusky except for white to cream or orange-tinged pelvics and clear pectorals. There are some reports of fins being almost fluorescent purple.
Size
Reaches 1.2 m and 45.0 kg, possibly to 90 kg. The world, all-tackle angling record weighed 24.72 kg and was caught in 1972 at Nickajack Lake, Tennessee. A 9.35 kg fish was caught in the French River, Ontario. A 22 pound fish was caught at the base of the Rideau Falls in July 1961 (Hopkins, 1990b).
Found from Hudson Bay south to Guatemala, but not in Atlantic coast drainages south of the St. Lawrence River basin. This is the greatest range in latitude of any North American freshwater fish. In Canada from the upper St. Lawrence River basin, Ottawa River, at Abitibi in the James Bay drainage, in the Great Lakes except for Lake Superior, in Manitoba to Hudson Bay and in southwestern Saskatchewan. This species is known mostly from the Ottawa River in the NCR and, rarely, in the Rideau River with one specimen taken between the Bank and Bronson Street bridges in 1998 (Phelps et al. (2000). It is not commonly caught in the NCR, with less than 10 fish on record before 2004, but this may be misleading as it is based on scientific collections. It is probably quite common in the Ottawa River, being regularly caught by anglers at Petrie Island for example, and it is also present in the South Nation River (personal observations, August 2004).
Origin
This species entered the NCR from a Mississippian refugium.
Habitat
The favoured habitat of this species is large and shallow waters including both lakes and rivers. Both clear and turbid water is occupied. Freshwater Drums retire to deeper waters in winter and in summer are found at less than 10 m in Lake Erie for example. Its preferred temperature is 26.5°C.
Age and Growth
Females attain larger sizes than males and grow faster after the first 5 years. Males can be mature at 2 years and all are mature at 6. Females mature 1 year later than males. Most drums in Lake Erie are 3-8 years old but they live at least 17 years.
Food
Food is insects, crustaceans such as crayfish, molluscs and fish. River populations are said to favour clams and snails, which are crushed by the large molariform throat teeth, but molluscs are not as significant a part of the diet as might be expected from the specialised throat teeth. However, the exotic zebra mussels are eaten by drums which may help control this pest. The young eat crustaceans and aquatic insects.

Reproduction
Spawning occurs from June to September in southern Canada but comparatively little is known about it. It may take place over sand or mud in shallows or in open water far from shore. Temperatures in mid- to late June are 20-23°C in Manitoba when mature fish are found. Comtois et al. (2006) report fish in reproductive stage V (spawning) in the lower Gatineau River from 3 May to 6 May at 9-10ºC but only two specimens were caught. Courtship is accompanied by the drumming sounds of the males, especially in the evening, and has been likened to the sound of rumbling traffic at a distance. Up to 686,000 eggs of 1.7 mm diameter are found in each female. Eggs float to the surface, an unusual feature in freshwater Canadian freshwater fishes.
Importance
An angler catch of 129,327 drums was made in Ontario waters of Lake Erie from June to August 1978. It is also a commercially important species and is marketed as "white perch". Over half a million kg have been caught annually from Lake Erie in Canadian waters. Small (1883) and Small and Lett (1884; 1885) noted that this species had been abundant on Ottawa markets, had declined and was again increasing. The flesh is firm and flavourful and is delicious when smoked. The Ontario Ministry of Natural Resources publishes a print and on-line "Guide to Eating Ontario Sport Fish" (www.mnr.gov.on.ca/MNR/) and has advisory limits for eating this species in the South Nation River. As these limits are apt to change, anglers consuming this fish should consult the most recent version.
This species is the only known host for the glochidia (= larvae) of the Pink Heelsplitter, a freshwater mussel (Potamilus alatus)(A. Martel, personal communication, 2002).
The following list includes all the species known to occur in the NCR.
The names follow the American Fisheries Society list (Nelson et al., 2004).
See also Desroches (2009) and Brisson et al. (2009) for some proposed
French name changes. One species that may have reached the NCR in the
past is the American Shad (Alose savoureuse, Alosa sapidissima (Wilson,
1811)). The construction of the dam at Carillon on the Ottawa River below the
NCR blocked migrations of this species (MacDonald, 1938; Provost et al., 1984). Some
species records have been discredited, such as one for Lepomis megalotis
(Rafinesque, 1820) in Lee et al. (1980) which was an error of labeling
(Coad, 1985a) but note that Chabot and Caron (1996) map this fish from the
Ottawa River (voucher specimens are needed). Other records may also be in error and have not been confirmed by
specimens, or a listing may include species for a trans-boundary drainage yet to
be found in the NCR, e.g. the cyprinids Lythrurus
umbratilis and Rhinichthys atratulus
from the Kemptville Creek drainage in Schueler et al. (1996). Certain species are excluded as they do not establish permanent
populations. These are the oscar (Astronotus ocellatus (Agassiz,
1831)) and a pacu or piranha, both discarded aquarium fishes (Anonymous, 1999c;
Barker, 1999; Bonenfant, 1999; Duquette, 1999; Renaud and Berkowitz, 1999;
Renaud and Phelps, 1999; 2001). A tilapia, believed to be the Blue Tilapia (Oreochromis
aureus (Steindachner, 1864)), has been found in a stormwater retention pond
in Ottawa (Nicholas Mandrak, pers. comm., 23 June 2010) and again does not
probably represent an established population. The blind
cave fish from Carleton University groundwater was an April Fool's joke (Anonymous,
1994). Concern has been expressed about the live sale for food of Asian carp
(probably Bighead Carp, Hypophthalmichthys nobilis (Richardson, 1845)) in
Ottawa stores. There is potential for escapes into local waters where this fish
could seriously affect native species (Singer, 2003).
|
Scientific name/Nom scientifique |
English name/Nom anglais |
French name/Nom français |
|
Petromyzontidae |
Lampreys |
Lamproies |
|
1. Ichthyomyzon castaneus Girard, 1858 |
Chestnut Lamprey |
Lamproie brune |
|
2. Ichthyomyzon fossor Reighard and Cummins, 1916 |
Northern Brook Lamprey |
Lamproie du nord |
|
3. Ichthyomyzon unicuspis Hubbs and Trautman, 1937 |
Silver Lamprey |
Lamproie argentée |
|
4. Lampetra appendix (DeKay,1842) |
American Brook Lamprey |
Lamproie de l'est |
|
Acipenseridae |
Sturgeons |
Esturgeons |
|
1. Acipenser fulvescens Rafinesque, 1817 |
Lake Sturgeon |
Esturgeon jaune |
|
Lepisosteidae |
Gars |
Lépisostés |
|
1. Lepisosteus osseus (Linnaeus, 1758) |
Longnose Gar |
Lépisosté osseux |
|
Amiidae |
Bowfins |
Poissons-castors |
|
1. Amia calva Linnaeus, 1766 |
Bowfin |
Poisson-castor |
|
Hiodontidae |
Mooneyes |
Laquaiches |
|
1. Hiodon tergisus Lesueur, 1818 |
Mooneye |
Laquaiche argentée |
|
Anguillidae |
Freshwater Eels |
Anguilles d'eau douce |
|
1. Anguilla rostrata (Lesueur, 1817) |
American Eel |
Anguille d'Amérique |
|
Clupeidae |
Herrings |
Harengs |
|
1. Alosa pseudoharengus (Wilson, 1811) |
Alewife |
Gasparaeau |
|
Cyprinidae |
Carps and Minnows |
Carpes et Ménés |
|
1. Carassius auratus (Linnaeus, 1758) |
Goldfish |
Carassin |
|
2. Cyprinella spiloptera (Cope, 1867) |
Spotfin Shiner |
Méné bleu |
|
3. Cyprinus carpio Linnaeus, 1758 |
Common Carp |
Carpe |
|
4. Hybognathus hankinsoni Hubbs, 1929 |
Brassy Minnow |
Méné laiton |
|
5. Hybognathus regius Girard, 1856 |
Eastern Silvery Minnow |
Méné d'argent |
|
6. Luxilus cornutus (Mitchill, 1817) |
Common Shiner |
Méné à nageoires rouges |
|
7. Margariscus margarita (Cope, 1867) |
Pearl Dace |
Mulet perlé |
|
8. Nocomis biguttatus (Kirtland, 1840) |
Hornyhead Chub | Tête à taches rouges |
|
9. Notemigonus crysoleucas (Mitchill, 1814) |
Golden Shiner |
Méné jaune |
|
10. Notropis atherinoides Rafinesque, 1818 |
Emerald Shiner |
Méné émeraude |
|
11. Notropis heterodon (Cope, 1865) |
Blackchin Shiner |
Menton noir |
|
12. Notropis heterolepis Eigenmann and Eigenmann, 1893 |
Blacknose Shiner |
Museau noir |
|
13. Notropis hudsonius (Clinton, 1824) |
Spottail Shiner |
Queue à tache noir |
|
14. Notropis rubellus (Agassiz, 1850) |
Rosyface Shiner |
Tête rose |
|
15. Notropis stramineus (Cope, 1865) |
Sand Shiner |
Méné paille |
|
16. Notropis volucellus (Cope, 1865) |
Mimic Shiner |
Méné pâle |
|
17. Phoxinus eos (Cope, 1862) |
Northern Redbelly Dace |
Ventre rouge du nord |
|
18. Phoxinus neogaeus Cope, 1867 |
Finescale Dace |
Ventre citron |
|
19. Pimephales notatus (Rafinesque, 1820) |
Bluntnose Minnow |
Ventre-pourri |
|
20. Pimephales promelas Rafinesque, 1820 |
Fathead Minnow |
Tête-de-boule |
|
21. Rhinichthys cataractae (Valenciennes, 1842) |
Longnose Dace |
Naseux de rapides |
|
22. Semotilus atromaculatus (Mitchill, 1818) |
Creek Chub |
Mulet à cornes |
|
23. Semotilus corporalis (Mitchill, 1817) |
Fallfish |
Ouitouche |
|
Catostomidae |
Suckers |
Catostomes |
|
1. Carpiodes cyprinus (Lesueur, 1817) |
Quillback |
Couette |
|
2. Catostomus catostomus (Forster, 1773) |
Longnose Sucker |
Meunier rouge |
|
3. Catostomus commersonii (Lacepède, 1803) |
White Sucker |
Meunier noir |
|
4. Moxostoma anisurum (Rafinesque, 1820) |
Silver Redhorse |
Chevalier blanc |
|
5. Moxostoma carinatum (Cope, 1870) |
River Redhorse |
Chevalier de rivière |
|
6. Moxostoma macrolepidotum (Lesueur, 1817) |
Shorthead Redhorse |
Chevalier rouge |
|
7. Moxostoma valenciennesi Jordan, 1885 |
Greater Redhorse |
Chevalier jaune |
|
Ictaluridae |
North American Catfishes |
Barbottes et Barbues |
|
1. Ameiurus natalis (Lesueur, 1819) |
Yellow Bullhead |
Barbotte jaune |
|
2. Ameiurus nebulosus (Lesueur, 1819) |
Brown Bullhead |
Barbotte brune |
|
3. Ictalurus punctatus (Rafinesque, 1818) |
Channel Catfish |
Barbue de rivière |
|
4. Noturus flavus Rafinesque, 1818 |
Stonecat |
Barbotte de rapides |
|
5. Noturus gyrinus (Mitchill, 1817) |
Tadpole Madtom |
Chat-fou brun |
|
6. Noturus insignis (Richardson, 1836) |
Margined Madtom |
Chat-fou liséré |
|
Esocidae |
Pikes |
Brochets |
|
1. Esox lucius Linnaeus, 1758 |
Northern Pike |
Grand brochet |
|
2. Esox masquinongy Mitchill, 1824 |
Muskellunge |
Maskinongé |
|
Umbridae |
Mudminnows |
Umbres |
|
1. Umbra limi (Kirtland, 1840) |
Central Mudminnow |
Umbre de vase |
|
Osmeridae |
Smelts |
Éperlans |
|
1. Osmerus mordax (Mitchill, 1814) |
Rainbow Smelt |
Éperlan arc-en-ciel |
|
2. Osmerus spectrum Cope, 1870 |
Pygmy Smelt |
Éperlan nain |
|
Salmonidae |
Trouts and Salmons |
Truites et Saumons |
|
1. Coregonus artedi Lesueur, 1818 |
Cisco |
Cisco de lac |
|
2. Coregonus clupeaformis (Mitchill, 1818) |
Lake Whitefish |
Grande corégone |
|
3. Oncorhynchus clarkii (Richardson, 1836) |
Cutthroat Trout |
Truite fardée |
|
4. Oncorhynchus mykiss (Walbaum, 1792) |
Rainbow Trout |
Truite arc-en-ciel |
|
5. Salmo salar Linnaeus, 1758 |
Atlantic Salmon |
Saumon Atlantique |
|
6. Salmo trutta Linnaeus, 1758 |
Brown Trout |
Truite brune |
|
7. Salvelinus alpinus (Linnaeus, 1758) |
Arctic Charr |
Omble chevalier |
|
8. Salvelinus fontinalis (Mitchill, 1814) |
Brook Trout |
Omble de fontaine |
|
9. Salvelinus namaycush (Walbaum, 1792) |
Lake Trout |
Touladi |
|
Percopsidae |
Trout-perches |
Omiscos |
|
1. Percopsis omiscomaycus (Walbaum, 1792) |
Trout-perch |
Omisco |
|
Gadidae |
Cods |
Morues |
|
1. Lota lota (Linnaeus, 1758) |
Burbot |
Lotte |
|
Atherinopsidae |
New World Silversides |
Poissons d'argent |
|
1. Labidesthes sicculus (Cope, 1865) |
Brook Silverside |
Crayon d'argent |
|
Fundulidae |
Topminnows |
Fondules |
|
1. Fundulus diaphanus (Lesueur, 1817) |
Banded Killifish |
Fondule barré |
|
Gasterosteidae |
Sticklebacks |
Épinoches |
|
1. Culaea inconstans (Kirtland, 1840) |
Brook Stickleback |
Épinoche à cinq épines |
|
2. Gasterosteus aculeatus Linnaeus, 1758 |
Threespine Stickleback |
Épinoche à trois épines |
|
3. Pungitius pungitius (Linnaeus, 1758) |
Ninespine Stickleback |
Épinoche à neuf épines |
|
Cottidae |
Sculpins |
Chabots |
|
1. Cottus bairdii Girard, 1850 |
Mottled Sculpin |
Chabot tacheté |
|
Centrarchidae |
Sunfishes |
Achigans et Crapets |
|
1. Ambloplites rupestris (Rafinesque, 1817) |
Rock Bass |
Crapet de roche |
|
2. Lepomis gibbosus (Linnaeus, 1758) |
Pumpkinseed |
Crapet-soleil |
|
3. Lepomis macrochirus Rafinesque, 1819 |
Bluegill |
Crapet arlequin |
|
4. Micropterus dolomieu Lacepède, 1802 |
Smallmouth Bass |
Achigan à petite bouche |
|
5. Micropterus salmoides (Lacepède, 1802) |
Largemouth Bass |
Achigan à grande bouche |
|
6. Pomoxis nigromaculatus (Lesueur, 1829) |
Black Crappie |
Marigane noir |
|
Percidae |
Perches |
Perches et Dards |
|
1. Etheostoma exile (Girard, 1859) |
Iowa Darter |
Dard à ventre jaune |
|
2. Etheostoma flabellare Rafinesque, 1819 |
Fantail Darter |
Dard barré |
|
3. Etheostoma nigrum Rafinesque, 1820 |
Johnny Darter |
Raseux-de-terre noir |
|
4. Etheostoma olmstedi Storer, 1842 |
Tessellated Darter |
Raseux-de-terre gris |
|
5. Perca flavescens (Mitchill, 1814) |
Yellow Perch |
Perchaude |
|
6. Percina caprodes (Rafinesque, 1818) |
Logperch |
Fouille-roche zébré |
|
7. Percina copelandi (Jordan, 1877) |
Channel Darter |
Fouille-roche gris |
|
8. Sander canadensis (Griffith and Smith, 1834) |
Sauger |
Doré noir |
|
9. Sander vitreus (Mitchill, 1818) |
Walleye |
Doré jaune |
|
Sciaenidae |
Drums and Croakers |
Tambours |
|
1. Aplodinotus grunniens Rafinesque, 1819 |
Freshwater Drum |
Malachigan |
Introduction
Certain species in the NCR are very distinctive and recognisable at a glance, others are superficially similar. These keys provide a structured means to identify all the species, especially where the student of these fishes is new to the fauna. They first appeared in Coad (2001).
The first key presented here is to the families of fishes. Where there is only a single species in the family, that species will be identified here. Separate keys are given for families with more than one species.
Three species have been added to the list, that were not recorded in Coad (1986b), the most recent listing of this ichthyofauna. These are Ichthyomyzon castaneus Girard, 1858 (Chestnut Lamprey/Lamproie Brune) documented by Renaud and de Ville (2000); Carassius auratus (Linnaeus, 1758) (Goldfish/Carassin) - Goldfish have been introduced to several ponds in the NCR and seem to survive the winter but they may revert to the wild type with camouflage colouring, losing the typical "gold" colours; and Nocomis biguttatus (Kirtland, 1840) (Hornyhead Chub/Tête à taches rouges) documented by Coad and Alfonso (in prep.).
Three "species" are not included. The Oscar, a South American member of the family Cichlidae (Astronotus ocellatus (Agassiz in Spix and Agassiz, 1831)) caught in the Rideau Canal in 1999, was evidently a released aquarium specimen which became a minor media star (Anonymous, 1999b; Barker, 1999; Bonenfant A.-M., 1999; Duquette, 1999; Renaud and Phelps, 1999; Renaud and Berkowitz, 1999). A 12-14 inch fish with piranha-like teeth was found dead on the Queen Elizabeth Driveway shoreline, Dow's Lake by Hedrik Wachelka of Muskies Canada in 1994. It was observed on 23 October after the water was drained and the photograph is possibly of a pacu (Colossoma bidens (Spix and Agassiz, 1829)), another South American fish of the family Characidae, or even a piranha, and also most probably an aquarium release (Renaud and Phelps, 2001). The blind fish from groundwater at Carleton University was a hoax; the article appeared just in time for April Fool's Day (Anonymous, 1994).
Notes on the Keys
The counts and measurements given in the keys are for each species over its whole distribution: the ranges for these characters are likely to be less extensive within the restricted geographical area of the NCR.
Some characters are best examined under a microscope, require dissection and/or require some skill to observe accurately, e.g., gill raker counts, pharyngeal tooth structure. Species can often be distinguished on colour alone but colour does vary with season, by individual, by sex of the specimen, and by preservation state if live specimens are not at hand. Colour can be misleading and countable or readily observed characters are best.
A knowledge of fish anatomy and terminology of body parts is necessary to understand the keys. Some structures are briefly described where they first appear in these keys and are in bold text. The Figures also illustrate structures used in identification. Generally, closely related fish of equal size (and age) should be compared if at all possible as there are changes in characters with growth, e.g., body proportions, gill raker length. The collections of the Canadian Museum of Nature on Pink Road in Gatineau (formerly Aylmer) contain a wide variety of specimens of all National Capital Region species and these can be examined with permission of the Vertebrate Collection Manager (see www.nature.ca for contact information).
Summary and simplified distributional information is given after each species and some more recent information is included too (see Bibliography for recent works and Species Accounts for more details). "Widespread" indicates that the fish is found in several to many diverse ecological and geographical habitats and localities, usually in both Ontario and Québec, while river localities indicate the species is restricted to those rivers and/or lower reaches of tributaries. Further field work may expand the distributions given here although restricted distributions are usually indicative of narrow tolerance for environmental conditions, e.g., the Lake Sturgeon is not likely to be found outside the large Ottawa River and lower reaches of large tributaries; salmonids generally prefer cooler waters and are most common on the Québec side; the Spotfin Shiner is known only from a few localities such as Bear Creek.
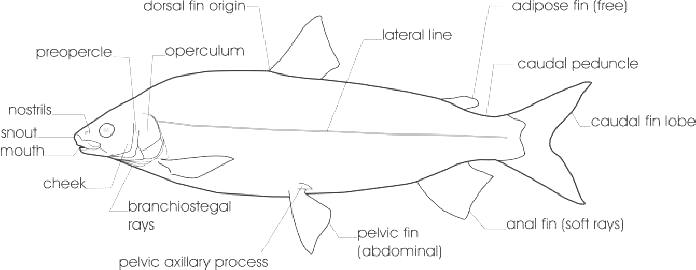
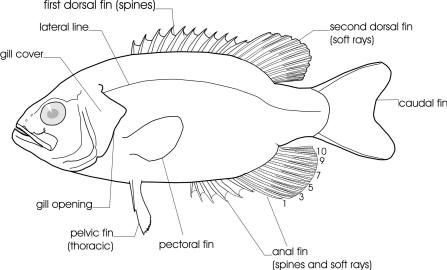
gill arch with rakers to left
Key to Families
1. 7 pairs of pored gill openings; no pectoral or pelvic fins; a median
nostril; "mouth" a rounded, suctorial disc in adults, or a fleshy oral
hood in larvae (or ammocoetes) = Petromyzontidae (Lampreys/Lamproies)
1 external gill opening on each side of head; at least pectoral fins present and usually pelvic fins too; paired nostrils; mouth bounded by upper and lower jaws -->
2. Caudal fin lobes strongly asymmetrical, the upper lobe much larger than the lower and containing the vertebral column; 2 pairs of barbels in front of subterminal (under head) mouth; 5 rows of bony plates along body = Acipenseridae (Sturgeons/Esturgeons) Acipenser fulvescens (Lake Sturgeon/Esturgeon jaune) [Ottawa and Gatineau rivers]
Caudal fin lobes mostly symmetrical externally (when present), no extensive presence of vertebral column in lobes; barbels if present not anterior to mouth; body without 5 rows of plates, with regular scales, or no scales --> 3
3. Gular plate present (large bony structure on lower surface of head between jaws); anterior nostrils tubular and near snout tip = Amiidae (Bowfins/Poissons-castors) Amia calva (Bowfin/Poisson-castor) [Ottawa River]
No gular plate; anterior nostrils not as above --> 4
4. No pelvic fins; body extremely elongate; dorsal, caudal and anal fins continuous; scales so small as not to be readily visible = Anguillidae (Freshwater Eels/Anguilles d'eau douce) Anguilla rostrata (American Eel/Anguille d'Amérique) [Ottawa, Gatineau and Mississippi rivers]
Pelvic fins present; body not extremely elongate; dorsal, caudal and anal fins separate; scales absent, or present and obvious --> 5
5. Scales like armour, exceptionally thick, close fitting, non-overlapping and diamond-shaped; jaws very elongate and narrow, longer than rest of head, armed with teeth = Lepisosteidae (Gars/Lépisostés) Lepisosteus osseus (Longnose Gar/Lépisosté osseux) [Ottawa River]
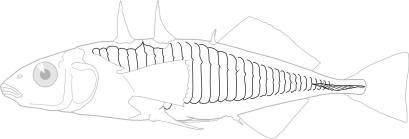
Scales thin and overlapping, or absent; jaws not as above --> 6
6. Second spine of anal fin massive; lateral line runs to end of caudal fin; head canals greatly enlarged = Sciaenidae (Drums and Croakers/Tambours) Aplodinotus grunniens (Freshwater Drum/Malachigan) [Ottawa, Gatineau and Rideau rivers]
Anal fin without spine, or spine not massive; lateral line when present not extending to end of caudal fin; head canals not greatly enlarged --> 7
7. Soft dorsal fin preceded by 2-12 isolated spines, not joined by a membrane = Gasterosteidae (Sticklebacks/Épinoches)
Dorsal fin without spines or, if spines present, not isolated but joined by a membrane --> 8
8. Single barbel on chin tip; pelvic fins clearly inserted anterior to pectoral fin origin = Gadidae (Cods/Morues) Lota lota (Burbot/Lotte) [Ottawa, Gatineau and Mississippi rivers]
No chin barbel (other barbels may be present on the jaws); pelvic fins under or behind pectoral fin origin --> 9
9. Adipose fin present --> 10
Adipose fin absent --> 13
10. Scales absent; 4 pairs of long barbels present; very strong spine-like rays in the dorsal and pectoral fins = Ictaluridae (North American Catfishes/Barbottes et Barbues)
Scales present; no barbels, or barbels not in 4 pairs; no very strong fin spines --> 11
11. Pelvic fin anterior, overlapped by pectoral fin; mouth under head; scales weakly ctenoid (minute spines at posterior margin); teeth very fine, difficult to see = Percopsidae (Trout-perches/Omiscos) Percopsis omiscomaycus (Trout-perch/Omisco) [widespread]
Pelvic fin more posterior on abdomen, not overlapped by pectoral fin; mouth at tip of head; scales cycloid (no minute spines); teeth small to large but easy to see --> 12
12. Pelvic axillary process (flap at fin base) absent; pelvic fin origin below or in front of dorsal fin origin = Osmeridae (Smelts/Éperlans)
Pelvic axillary process present; pelvic fin origin behind dorsal fin origin = Salmonidae (Trouts and Salmons/Truites et Saumons)
13. Pelvic fins abdominal (about mid-body, behind the pectoral fin tip); one dorsal fin; no true fin spines (Cyprinus carpio and Carassius auratus have a single anterior spine-like ray in the dorsal and anal fins) --> 14
Pelvic fins thoracic (anterior, under pectoral fin); two dorsal fins or, if one fin, then several spines anteriorly --> 21
14. Gill openings narrow (gill membranes joined to isthmus, the area between the gill openings on the lower head surface - right below) --> 15
Gill openings wide (gill membranes not joined to isthmus - left below) --> 16
15. Lips thick and fleshy; mouth under head; pharyngeal teeth (throat teeth, lying behind the gill arches and accessible via the gill opening by dissection) in a single row, numerous, more than 15; anal fin posterior, the distance between the posterior opercle edge to the anal fin origin clearly greater than the distance from anal fin origin to the caudal fin base = Catostomidae (Suckers/Catostomes)
Lips thin; mouth usually at tip of head (under head in Rhinichthys cataractae but this species has 2 rows of pharyngeal teeth, 1-4 in each row); pharyngeal teeth in 0-3 rows but never more than 5 in a row; anal fin more anterior, the distance between the posterior opercle edge to the anal fin origin marginally greater than the distance from anal fin origin to the caudal fin base = Cyprinidae (Carps and Minnows/Carpes et Ménés)
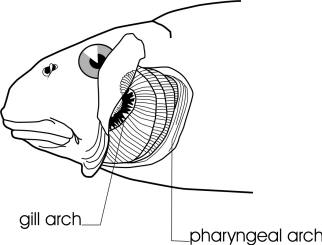
16. Scales on top of head --> 17
Top of head scaleless --> 20
17. Groove between upper lip and snout (premaxillaries protractile - anteriormost bones of upper jaw can extend) = 18
No groove (premaxillaries not protractile) --> 19
18. One dorsal fin; anal fin with 9-13 rays, no spine = Fundulidae (Topminnows/Fondules) Fundulus diaphanus (Banded Killifish/Fondule barré) [widespread]
Two dorsal fins (the first small and easily missed) over the anal fin; anal fin with 20-27 rays after a spine = Atherinopsidae (New World Silversides/Poissons d'Argent) Labidesthes sicculus (Brook Silverside/Crayon d'argent) [Ottawa and Rideau rivers]
19. Snout a duck-bill shape; caudal fin forked; strong teeth; lateral line scales 105-176 = Esocidae (Pikes/Brochets)
Snout normal-shaped; caudal fin rounded; teeth minute; lateral line scales 30-37 = Umbridae (Mudminnows/Umbres) Umbra limi (Central Mudminnow/Umbre de vase) [widespread]
20. Dorsal fin over pelvic fins; no obvious teeth on tongue; lateral line absent; belly with scutes (sharp scales); gill rakers (bony projections on the inner side of the gill arch) numerous and elongate; anal fin rays 15-21; nostrils near eye = Clupeidae (Herrings/Harengs) Alosa pseudoharengus (Alewife/Gaspareau) [Rideau River]
Dorsal fin over anal fin; strong teeth present on tongue; lateral line present; no belly scutes; gill rakers few and knob-like; anal fin rays 26-31; nostrils near snout tip = Hiodontidae (Mooneyes/Laquaiches) Hiodon tergisus (Mooneye/Laquaiche argentée) [Ottawa, Gatineau and Mississippi rivers]
21. Body mostly naked (some minute prickles), no scales; pectoral fin large and fan-shaped; head broad and flattened = Cottidae (Sculpins/Chabots) Cottus bairdii (Mottled Sculpin/Chabot tacheté) [widespread]
Body scaled; pectoral fin not as large; head not broad and flattened --> 22
22. One to two anal fin spines; two dorsal fins at most slightly joined at base = Percidae (Perches/Perches et Dards)
Three or more anal fin spines; one dorsal fin (comprising joined spiny and soft parts except almost divided in two at base in Micropterus salmoides) = Centrarchidae (Sunfishes/Achigans et Crapets)
Key to Adult Petromyzontidae (Lampreys/Lamproies)
Adults have teeth in a sucking disc which is lined around the rim by fimbriae (short, thread-like filaments), and they have well-developed eyes. They swim free, or are attached as external parasites to other fish, or may hide under rocks and debris.
1. Two distinct dorsal fins; trunk myomeres (muscle blocks) 63-74 = Lampetra appendix (American Brook Lamprey/Lamproie de l'est) [Ottawa and Gatineau rivers]
Dorsal fin single with at most a slight dip at mid-point, dip not reaching body; trunk myomeres 47-58 --> 2
2. Teeth small and blunt; lateral line organs lacking pigmentation; gut shrivelled, diameter minute; small, length up to about 25.4 cm and often 15 cm or less = Ichthyomyzon fossor (Northern Brook Lamprey/Lamproie du nord)[Ottawa River]
Teeth large, sharp and curved; lateral line organs obviously pigmented; gut well-developed (but atrophies at spawning); larger, length up to about 38 cm --> 3
3. Circumoral teeth usually unicuspid; trunk myomeres 47-55 (usually 49-52, mode 51) = Ichthyomyzon unicuspis (Silver Lamprey/Lamproie argentée) [Ottawa and Gatineau rivers]
Circumoral teeth usually bicuspid; trunk myomeres 49-56 (usually 51-54, mode 53) = Ichthyomyzon castaneus (Chestnut Lamprey/Lamproie brune) [Brewery Creek]
Key to Larval Petromyzontidae (Lampreys/Lamproies)
Note that this key may not work for smaller ammocoetes (<105 mm for the Chestnut Lamprey, i.e. presence of the black lateral line organs means the ammocoete is a Chestnut Lamprey but absence in smaller specimens does not mean it is not), and the distinction of unicuspis and fossor is not possible on pigmentation patterns, at least for Great Lakes fish (Neave, 2004; Neave et al., 2007).
Larval lampreys or ammocoetes lack teeth and have a fleshy hood enclosing the oral opening and eyes are weakly developed. They live buried in sediment with the head protruding and are not usually free-swimming.
1. Two distinct dorsal fins; trunk myomeres 63-74 = Lampetra appendix (American Brook Lamprey/Lamproie de l'est)
Dorsal fin single with at most a slight dip at mid-point, dip not reaching body; trunk myomeres 47-58 --> 2
2. Lateral line organs black = Ichthyomyzon castaneus (Chestnut Lamprey/Lamproie brune)
Lateral line organs unpigmented --> 3
3. Caudal fin and head heavily pigmented; a whitish band above the gill openings is about 3 mm deep = Ichthyomyzon unicuspis (Silver Lamprey/Lamproie argentée)
Caudal fin and head weakly pigmented; a whitish band above the gill openings is about 1 mm deep = Ichthyomyzon fossor (Northern Brook Lamprey/Lamproie du nord)
Key to Cyprinidae (Carps and Minnows/Carpes et Ménés)
1. Dorsal and anal fins with a strong, serrated, anterior spine-like ray; dorsal fin branched rays 14-23 --> 2
Dorsal and anal fins without a spine-like ray; dorsal fin branched rays 6-8 --> 3
2. Two pairs of barbels; total gill rakers 21-27; pharyngeal teeth in three rows with usually 1,1,3 teeth on each side = Cyprinus carpio (Common Carp/Carpe) [Ottawa and Rideau rivers]
No barbels; total gill rakers 37-50; pharyngeal teeth in one row with usually 4 teeth on each side = Carassius auratus (Goldfish/Carassin) [various ponds in Ontario]
3. No groove between the snout and lip (premaxillaries not protractile, frenum present); snout projects far over ventral mouth; barbel at mouth corner (note spawning male Pimephales notatus develop a barbel-like structure at the rear tip of the upper jaw) = Rhinichthys cataractae (Longnose Dace/Naseux de rapides) [widespread]
A groove between the snout and lip (premaxillaries protractile); snout not markedly projecting and mouth at head tip; no barbel at mouth corner --> 4
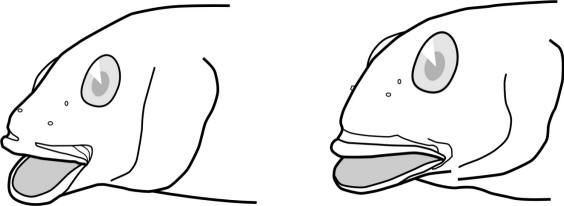
4. Small, short, flat, and often triangular barbel present in the groove between upper lip and snout, just anterior to mouth corner (often absent on one or both sides in Margariscus margarita, absent on both sides in 23% of over 1000 individuals from across North America; usually present in the other two species and in some fish from large samples of all species; if the barbel is absent on both sides, Margariscus margarita is distinguished by the high scale count in a complete lateral line, short s-shaped gut, pharyngeal teeth in main rows 5-4, silvery peritoneum (lining of body cavity, belly must be cut open to see), small mouth and lack of unique characters listed for other carp family members) --> 5
No such barbel --> 7
5. Lateral line scales 43-50; flank scales each with an anterior patch of pigment but overall colour is bright silvery = Semotilus corporalis (Fallfish/Ouitouche) [widespread]
Lateral line scales 46-79, usually 52-75; flank scales without an anterior patch of pigment, overall colour not bright silvery --> 6
6. Lateral line scales 47-66, usually 52-63; distinctive black spot at anterior dorsal fin base; body not spotted, scales outlined with pigment; mouth large, corner at level of front of eye or behind = Semotilus atromaculatus (Creek Chub/Mulet à cornes) [widespread]
Lateral line scales 46-79, usually 65-75; no black spot at anterior dorsal fin base; body usually appears spotted or speckled black and brown; mouth small, corner not reaching level of front of eye = Margariscus margarita (Pearl Dace/Mulet perlé) [widespread]
7. Lateral line scales 63 or more --> 8
Lateral line scales 57 or less --> 9
8. Peritoneum black (if silvery with dark speckles, flap-like barbel may have been missed or is absent --> 5), mouth small, extending to, or just past, the nostrils; 2 extra major loops in the gut; a stripe or series of spots between a dark upper flank stripe and the back; pharyngeal teeth in one row = *Phoxinus eos (Northern Redbelly Dace/Ventre rouge du nord) [widespread]
Peritoneum black (if silvery with dark speckles, flap-like barbel may have been missed or is absent --> 5), mouth large, extending to, or just past, the anterior eye margin; short s-shaped gut; no second stripe or spots as above; pharyngeal teeth in two rows = *Phoxinus neogaeus (Finescale Dace/Ventre citron) [widespread]
* Note: These two species commonly hybridize and diploid and triploid hybrids are reported from the NCR in Hawley (= Holly) Lake, Québec. Hybrids may occur in the absence of one parent species and are mostly female clones (Dawley et al., 1987; Dawley and Goddard, 1988). Character states in the key may not allow distinction of species.
9. Lateral line strongly decurved with 39-57 scales; naked fleshy keel between the pelvic fins and the anus on the belly midline; anal fin branched rays 7-18, usually 11-13 = Notemigonus crysoleucas (Golden Shiner/Méné jaune) [widespread]
Lateral line not strongly decurved; belly scaled at midline, no naked fleshy keel; anal fin branched rays usually 10 or less --> 10
10. Second dorsal fin unbranched ray short and stout, separated from next ray by a relatively wide membrane in adults (first ray is minute and difficult to see); back flattened; dark to dusky blotch at anterior dorsal fin above base in adults; scales in front of dorsal fin on back smaller and more crowded than flank scales, not in regular rows --> 11
Second dorsal fin unbranched ray thin and splint-like, closely attached to next ray; back more rounded; no blotch above anterior dorsal fin base; scales in front of dorsal fin on back usually large and distinct, more like flank scales and in regular rows --> 12
11. Lateral line usually incomplete, not extending along whole flank; mouth at tip of body; caudal fin base spot faint to absent; body deep, depth less than 4.0 times in standard length (tip of snout to end of vertebral column or tail base); dorsal fin origin above or slightly anterior to pelvic fin origin; gill rakers 14 or more = Pimephales promelas (Fathead Minnow/Tête-de-boule) [widespread]
Lateral line complete; mouth under snout; caudal fin base spot well-developed; body more slender, more than 4.5 times in standard length; dorsal fin origin posterior to pelvic fin origin; gill rakers 11 or fewer = Pimephales notatus (Bluntnose Minnow/Ventre-pourri) [widespread]
12. Gut long with numerous coils; mouth almost horizontal, below tip of head, with a long diagonal fold above and below its corner --> 13
Gut short with an s-shaped loop; mouth oblique, at tip of head (below tip in Notropis hudsonius but without long diagonal fold --> 14
13. Colour brassy in adult, dull silver in young; dorsal fin margin rounded; 14-20 radii (rarely 11-12) on adult scales (radii are grooves radiating from the scale centre); usually 5-6 scales between lateral line and pelvic fin; no thin black line along flank = Hybognathus hankinsoni (Brassy Minnow/Méné laiton) [widespread]
Colour bright silvery; dorsal fin margin falcate (sickle-shaped) and pointed; 12 or less radii on adult scales (range 5-12); usually 4 scales between lateral line and pelvic fins; thin black line along flank lying partly over dark stripe = Hybognathus regius (Eastern Silvery Minnow/Méné d'argent) [Ottawa and Rideau rivers]
14. Barbel distinct, slender and present at angle of upper and lower jaw = Nocomis biguttatus (Hornyhead Chub/Tête à taches rouges)
No barbel --> 15
15. Anal fin branched rays 8-11, rarely 7 (sometimes 7 in Luxilus cornutus but this species has anterior lateral line scales more than twice as high as wide); pharyngeal tooth formula usually 2,4-4,2 --> 16
Anal fin branched rays 6-7, rarely 8; pharyngeal tooth formula usually 1,4-4,1 or 4-4 (2,4-4,2 in Notropis hudsonius but this species has a distinctive black spot on the caudal fin base) --> 18
16. Exposed part of anterior lateral line scales more than twice as high as wide; dorsal fin origin over or in front of the level of the pelvic fins insertion (posteriomost point of attachment of fin base) = Luxilus cornutus (Common Shiner/Méné à nageoires rouges) [widespread]
Exposed part of anterior lateral line scales about equal in width and depth (about twice as high as wide in Notropis volucellus but this species has black pigment around anus and anal fin base); dorsal fin origin behind the level of the pelvic fins insertion --> 17
17. Pectoral fin rays usually 15-17; snout length less than orbit (bony eye socket) diameter; anterior lateral line pores not outlined by pigment; predorsal stripe weak; flank pigment ends above lateral line anteriorly; distal anal fin margin concave; dorsal fin pointed, anterior ray tip reaching well beyond posterior ray tip when fin depressed = Notropis atherinoides (Emerald Shiner/Méné émeraude) [widespread]
Pectoral fin rays usually 11-14; snout length greater than orbit diameter in fish longer than 3.5 cm standard length; anterior lateral line pores outlined above and below by pigment spots giving a "stitched" appearance; predorsal stripe distinct; flank pigment ends at or below lateral line anteriorly; distal anal fin margin straight; dorsal fin not as pointed, anterior ray tip not reaching posterior ray tip when fin depressed = Notropis rubellus (Rosyface Shiner/tête rose) [widespread]
18. Dorsal fin with black blotch on last 2-4 membranes (may be weakly expressed or absent in young); snout pointed; eye small, less than one-quarter head length in adults (at least less than one-third) = Cyprinella spiloptera (Spotfin Shiner/méné bleu) [Bear Creek, Ottawa River]
Dorsal fin without posterior blotch; snout rounded; eye more than one-quarter head length in adults --> 19
19. Black spot at base of caudal fin large and obvious (may be obscured by silvery scales in live fish); dorsal and anal fins falcate = Notropis hudsonius (Spottail Shiner/Queue à tache noir) [widespread]
No obvious spot at caudal fin base; dorsal and anal fins convex or straight --> 20
20. Flank stripe strongly developed, extending onto head and to snout --> 21
Flank stripe weakly developed, not extending onto snout --> 22
21. Chin black (as is snout); mid-flank stripe often a strong black zig-zag; usually 2 rows of pharyngeal teeth = Notropis heterodon (Blackchin Shiner/Menton noir) [widespread]
Chin not black (but snout black); anterior mid-flank stripe composed of a series of crescents, tips pointing rearwards; usually 1 row of pharyngeal teeth = Notropis heterolepis (Blacknose Shiner/Museau noir) [widespread]
22. Area around anus and base of anal fin weakly pigmented; anal fin branched rays usually 6 (range 5-7); flank pigment not extending below lateral line; thin stripe on mid-line of back = Notropis stramineus (Sand Shiner/Méné paille) [Ottawa, Jock and Mississippi rivers]
Area around anus and base of anal fin black; anal fin branched rays usually 7 (range 7-8); flank pigment extends below lateral line; no distinct stripe on mid-line of back = Notropis volucellus (Mimic Shiner/Méné pâle) [widespread]
Key to Catostomidae (Suckers/Catostomes)
Adults
1. Dorsal fin long, with 22-32 major rays; anterior rays elongated into a high, sickle-shape = Carpiodes cyprinus (Quillback/Couette) [Ottawa and Gatineau rivers]
Dorsal fin short, with 9-18 major rays; anterior rays not markedly elongated --> 2
2. Scales small numbering 53 or more (usually 58 or more) in the lateral line; body rounded in cross section --> 3
Scales large numbering 48 or less in the lateral line; body compressed in cross section --> 4
3. Scales in lateral line 91-120 = Catostomus catostomus (Longnose Sucker/Meunier rouge) [Ottawa River, rare to absent]
Scales in lateral line 53-85 (usually 58-68) = Catostomus commersonii (White Sucker/Meunier noir) [widespread]
4. Scales around caudal peduncle (tail stem in front of tail fin) usually 15-16, rarely 12-13; dorsal fin distal margin usually strongly convex in adults (very slightly concave to straight in young); snout laterally mottled; mouth more terminal (terminal = at tip of snout) than other species = Moxostoma valenciennesi (Greater Redhorse/Chevalier jaune) [Ottawa, Mississippi and Rideau rivers, Bear Creek]
Scales around caudal peduncle usually 12-13, rarely 15-16; dorsal fin distal margin slightly convex (may be obviously convex in adult Moxostoma anisurum), straight, emarginate, or falcate; snout not mottled --> 5
5. Lower lip narrow, posterior margins of each half meet at an acute angle centrally; lower lip with transverse lines on folds or plicae, forming irregular-sized, oblong papillae; lower lip narrows abruptly before joining upper lip; maximum body depth into body length (head tip to end of scales) 3.5 times or less; dorsal fin rays usually 14-15 (range 12-17); caudal fin dusky to silver and all fins without red colour; flank blotches pale to absent = Moxostoma anisurum (Silver Redhorse/Chevalier blanc) [widespread]
Lower lip broader, posterior margins of each half meet at an obtuse angle centrally, may be almost straight (rarely acute at about 80°); lower lip without transverse lines on folds or plicae (large, oval papillae may be found posteriorly on the lower lip of large Moxostoma macrolepidotum); lower lip thick at corners; maximum body depth into body length (head tip to end of scales) 3.7 times or more; dorsal fin rays usually 13 or less; caudal fin pink to blood red (paler in young); 3-4 flank blotches distinct --> 6
6. Pharyngeal arch strongly developed with 6-9, large molar teeth on lower half of tooth row; distal dorsal fin margin rounded, straight, or slightly concave; long head, less than 4.3 times in standard length; mouth large; gill rakers with spots at base or on lower part of raker = Moxostoma carinatum (River Redhorse/Chevalier de rivière) [Ottawa, Gatineau and Mississippi rivers]
Pharyngeal arch not strongly developed, teeth comb- or blade-like and numerous, more than 11 on lower half of tooth row; distal dorsal fin margin falcate, or s-shaped; short head, 4.3-5.4 times in standard length; mouth small, not as wide as head; no gill raker spots = Moxostoma macrolepidotum (Shorthead Redhorse/Chevalier Rouge) [widespread]
Key to young Moxostoma (Redhorses/Suceurs)
(modified after Vachon (2003))
Young or juvenile Moxostoma (total length 35-150 mm) can be identified by the key below. These redhorses are particularly difficult to identify as young and Nathalie Vachon's careful study is based on fish from the Richelieu and St. Lawrence rivers in Québec but should be applicable to fish in the NCR. Characters in brackets are additional ones that will aid in identification but are not always unique to the species.
1. Scales around caudal peduncle (tail stem in front of tail fin) usually 15-16, rarely 12-13; supraorbital canals (pored, bony canals on the head running over the eye) not well-developed and not usually visible [pigmentation present and intense on the latero-ventral body, especially near the pectoral fins; relatively uniform pigmentation of the opercle or mostly concentrated on the upper half with a vertical row anteriorly; a relatively diffuse stripe behind the supratemporal canal across the occiput (top of the head at the back); dorsal fin very slightly concave or straight with 13-14, rarely 12, rays; body depth high to moderate; small melanophores diffusely spread on the body and snout giving a mottled aspect, very rarely forming 4 saddles (pigment in a saddle-shape over the back and down onto the sides); pharyngeal arches delicate with numerous comb-like teeth] = Moxostoma valenciennesi (Greater Redhorse/Chevalier jaune)
Scales around caudal peduncle usually 12-13, rarely 15-16; supraorbital canals moderately to strongly developed and visible --> 2
2. Supraorbital canals strongly developed, visible and straight in the anterior part from the snout to about mid-eye [pigmentation behind the supratemporal canal strong; large lower lip straight or slightly curved with obvious longitudinal ridges or plicae and often with deep transverse grooves; dorsal fin slightly concave with 13 rays, rarely 12, 14 or 15; body depth moderate to low; large melanophores distributed in 3-4 saddles; snout melanophores generally large; pharyngeal arches delicate with numerous comb-like teeth] = Moxostoma macrolepidotum (Shorthead Redhorse/Chevalier rouge)
Supraorbital canals moderately or strongly developed, visible and sinuous --> 3
3. Lower lip thin with an acute angle (90° or less) and with very fine longitudinal ridges [pigmentation behind the supratemporal canal not strong; supraorbital canal moderately or strongly developed, visible and sinuous especially in the anterior part with very visible pores; dorsal fin slightly concave or straight in larger fish with 14-16 rays, very rarely 13 or 17; body depth high or moderate; small melanophores evenly distributed on the body; pharyngeal arches delicate with numerous comb-like teeth] = Moxostoma anisurum (Silver Redhorse/Chevalier blanc)
Lower lip thin with an obtuse angle (>90°) and with realtively perceptible longitudinal ridges [pigmentation behind the supratemporal canal generally moderate; supraorbital canal moderately developed, visible and sinuous in the anterior part and occasionally in the posterior part; dorsal fin slightly concave with 12-14 rays, rarely 15; body elongate; small melanophores evenly distributed on the body, very rarely forming 3-4 saddles; black spot"(s) present at the base of the first gill raker; pharyngeal arches heavy with molariform lower teeth] = Moxostoma carinatum (River Redhorse/Chevalier de rivière)
Key to Ictaluridae (North American Catfishes/Barbottes et Barbues)
1. Adipose fin long, closely associated with the caudal fin, and attached to the back; small fishes, maximum size 11.5-31.2 cm, usually 17.3 cm or less --> 2
Adipose fin short, remote from the caudal fin, and posteriorly free from the back; large fishes, maximum size 46.5-120.2 cm --> 4
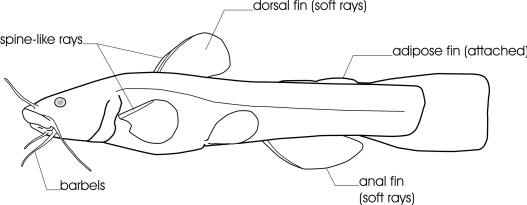
2. Premaxillary band of teeth (in anterior roof of mouth) with lateral processes extending backwards; posterior teeth of pectoral fin spine-like ray weaker than anterior teeth; back with pale areas behind head and at posterior end of dorsal fin; pectoral fin radials (bones in fin base - dissection or x-ray required) never fused = Noturus flavus (Stonecat/Barbotte des rapides) [Rideau River]
Premaxillary band of teeth without lateral processes extending backwards; posterior teeth of pectoral fin spine-like ray longer than anterior teeth or absent; back a uniform grey; pectoral fin radials usually fused --> 3
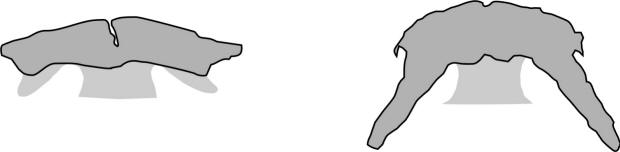
3. Body short, stout and tadpole-shaped; mouth terminal; pectoral spine-like ray without posterior teeth; branched pectoral rays 4-9 but usually 5-7 and modally 6; branched pelvic rays 4-9 but usually 7; typically 10 preoperculomandibular canal pores (canal runs from lower jaw onto the preopercle bone); dorsal, caudal and anal fins without a black distal margin; dark streak along mid-flank at intersection of dorsal and ventral muscle blocks = Noturus gyrinus (Tadpole Madtom/Chat-fou brun) [widespread]
Body elongate and slender; mouth subterminal; pectoral spine-like ray with posterior teeth; branched pectoral rays usually 8; branched pelvic rays usually 8; 11 preoperculomandibular canal pores; dorsal, caudal and anal fins with a black distal margin; no flank streak = Noturus insignis (Margined Madtom/Chat-Fou Liséré) [Gatineau Park and Gatineau River]
4. Caudal fin with a strong fork; almost continuous surface bony ridge on back between back of head and dorsal fin origin; mouth corner barbels more than 3 times longer than nostril barbels = Ictalurus punctatus (Channel Catfish/Barbue de rivière) [Ottawa, Gatineau and Mississippi rivers]
Caudal fin not forked, but truncate (square-cut) to rounded; back more fleshy, soft, depressible area before dorsal fin; mouth corner barbels about 2 times longer than nostril barbels --> 5
5. Chin barbels yellow to white or pinkish, upper barbels yellow to grey; anal fin touches caudal fin when pressed to the body; total anal rays 24-28; distance between lower jaw notch and tip of lower jaw much greater than distance between lower jaw notch and isthmus; fins (except pelvics and adipose) with black distal margins = Ameiurus natalis (Yellow Bullhead/Barbotte jaune) [Ottawa River]
All barbels brown to black (bases of lower ones may be pale in young); anal fin not reaching caudal fin when pressed to body; total anal rays 19-24; distance between lower jaw notch and tip of lower jaw about equal to distance between lower jaw notch and isthmus; no fins with black distal margins = Ameiurus nebulosus (Brown Bullhead/Barbotte brune) [widespread]
Key to Esocidae (Pikes/Brochets)
1. Submandibular pores 9-11 (usually 5 on each lower jaw); cheeks (the area between the eye and the preoperculum) fully scaled; flanks dark with lighter, wavy bars in young, or rows of light, bean-shaped spots in adults; no mid-dorsal light stripe (or a discontinuous one in young) = Esox lucius (Northern Pike/Grand brochet) [widespread]
Submandibular pores 12-20 (usually 6-10 on each lower jaw); cheeks only scaled on upper half; flanks light to silvery with darker spots, blotches or oblique bars; prominent mid-dorsal, light stripe in young = Esox masquinongy (Muskellunge/Maskinongé) [widespread]
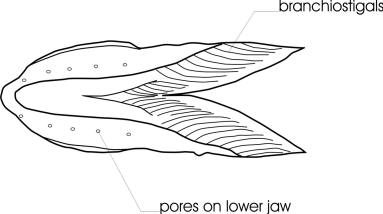
Key to Osmeridae (Smelts/Éperlans)
1. Total gill rakers 32-36 (usually 33-36); orbit diameter 4.4-6.5% of standard length; small, standard length less than 135 mm = Osmerus spectrum (Pygmy Smelt/Éperlan nain) [Meech Lake]
Total gill rakers 26-37 (usually 28-32); orbit diameter 7.0-11.3% of standard length; larger, standard length can exceed 200 mm = Osmerus mordax (Rainbow Smelt/Éperlan arc-en-ciel) [widespread]
Key to Salmonidae (Salmons/Saumons)
1. Teeth in lower jaw absent except in youngest fish; mouth small, lower jaw short, not extending back to mid-eye; scales large, 13 or less from dorsal fin origin to lateral line; caudal fin clearly forked; overall colour silvery to white --> 2
Teeth in lower jaw strong and conical; mouth large, lower jaw long, extending back to or past mid-eye; scales small, 19 or more from dorsal fin origin to lateral line; caudal fin usually truncate (obvious fork only in Salvelinus namaycush and Salmo salar); overall colour darker with spots and vermiculations --> 3
2. Mouth at tip of head; gill rakers 14-18 on upper arch; upper lip profile slopes forward in line with forehead (snout tip pointed viewed laterally) = Coregonus artedi (Cisco/Cisco de lac) [Québec lakes]
Mouth overhung by snout; gill rakers 7-12 on upper arch; upper lip profile vertical or bent back from line of forehead (snout tip rounded viewed laterally) = Coregonus clupeaformis (Lake Whitefish/Grande corégone) [Québec lakes]
3. Head and body with black spots (Salmo salar has red flank spots when young); pelvic and anal fins without white anterior margin; scales stand out; vomer bone (in centre of roof of mouth) flat, with teeth in 2 rows on shaft --> 4
Body with light spots of white, cream, pink, or red; pelvic and anal fins with conspicuous white anterior margin; scales inconspicuous; vomer bone deeper, without teeth on shaft --> 7
4. Caudal fin with radiating rows of black spots; body without red spots --> 5
Caudal fin lacking spots, if present not in rows; body may have red spots --> 6
5. Orange to red mark on each inner lower jaw (the "cutthroat"); small teeth on tongue base; no pink flank stripe (or if present, faint and copper-orange) = Oncorhynchus clarkii (Cutthroat Trout/Truite fardée) [Québec lakes and rivers]
No cutthroat mark; no basal tongue teeth; usually pink (or red to orange) flank stripe = Oncorhynchus mykiss (Rainbow Trout/Truite arc-en-ciel) [Québec lakes and rivers]
6. Opercular area with only 2-3 large spots; upper jaw not extending back much beyond eye (centre of eye in young); teeth on vomer bone weak; adipose fin without red or orange, adults lack red on flank (red present in young between parr marks and as a halo around dark spots in spawning fish); dark blotches on caudal fin if present not restricted to upper lobe; caudal fin deeply forked, medial rays less than half length marginal rays = Salmo salar (Atlantic Salmon/Saumon Atlantique) [Québec lakes]
Opercular area with many spots; upper jaw extending back well beyond eye (posterior half of eye in young); teeth on vomer bone strong; adipose fin often margined red or orange, adults often with rust-red flank spots with a blue halo; blotches on caudal fin if present only on upper lobe; caudal fin slightly forked, medial rays more than half the length of marginal rays = Salmo trutta (Brown Trout/Truite brune) [Québec lakes and rivers]
7. Caudal fin deeply forked; spots on flank sometimes irregular, bean-shaped whitish or cream (rarely orange); pyloric caeca (finger-like blind sacs attached to the junction of the stomach and intestine - see below) about 90-200; 7-12 parr marks (dark bars on flank of young fish), irregular, often interrupted, and narrow = Salvelinus namaycush (Lake Trout/Touladi) [Québec lakes, Gatineau River]
Caudal fin emarginate or square when spread out (may be forked in some Salvelinus alpinus populations); orange, pink or red spots on flank; pyloric caeca 13-75 --> 8
8. Dorsal and caudal fins and back with vermiculations (dark wavy lines); pelvic and anal fins with snow white leading edge set off by black behind; tip of lower jaw and roof of mouth black; flank spots usually surrounded by a blue halo; basibranchial teeth usually absent (on floor of mouth between gills); 8-10 regularly arranged parr marks along flank in young = Salvelinus fontinalis (Brook Trout/Omble de fontaine) - [widespread in Québec]
Dorsal and caudal fins without vermiculations; white leading edge not usually set off by black behind; tip of lower jaw and roof of mouth whitish; no blue halos around flank spots; basibranchial teeth present and numerous; 10-15 parr marks in young irregular and not clearly defined = Salvelinus alpinus (Arctic Char/Omble chevalier) [Québec lakes]
Key to Gasterosteidae (Sticklebacks/Épinoches)
1. Short dorsal fin spines 7-12 (usually 9), alternately sloping left and right = Pungitius pungitius (Ninespine Stickleback/Épinoche à neuf épines) [Québec lakes]
Short or long dorsal fin spines 3-7, in median line --> 2
2. Short dorsal fin spines 4-7; short pelvic spines (both less than eye diameter); scutes weakly developed = Culaea inconstans (Brook Stickleback/Épinoche à cinq épines) [widespread]
Long dorsal fin spines 3; long pelvic spines (both exceeding eye diameter); scutes (vertical bony plates on flanks) large = Gasterosteus aculeatus (Threespine Stickleback/Épinoche à trois épines) [widespread in Québec]
Key to Centrarchidae (Sunfishes/Crapets)
1. Dorsal fin spines 6-9, usually 7-8; length of whole anal and dorsal fin bases about equal = Pomoxis nigromaculatus (Black Crappie/Marigane noire) [Ottawa, Gatineau and Rideau rivers]
Dorsal fin spines 9-13; length of whole anal fin base 1.5-3.3 times in whole dorsal fin base length --> 2
2. Anal fin spines 5-7, usually 6, folding into a groove; length of anal fin base about 1.5 times in whole dorsal fin base length; 7-11 distinctive horizontal rows of spots below the lateral line; eye red = Ambloplites rupestris (Rock Bass/Crapet de roche) [widespread]
Anal fin spines 3, no basal groove; length of anal fin base 2.1-3.3 times in whole dorsal fin base length; no horizontal rows of spots below the lateral line --> 3
3. Lateral line scales small, 58-81; body rounded in cross section and elongate, maximum body depth 2.5-5.0 (usually 3.0 or more) times in body length from snout tip to end of scales --> 4
Lateral line scales large, 35-50; body compressed in cross section and deep, maximum body depth 1.7-3.0 (usually 2.5 or less) times in body length from snout tip to end of scales --> 5
4. Upper jaw extends back to about mid-pupil but not behind eye; lateral line scales 67-81; scales present on the bases of the soft dorsal and anal fin bases; young fish with 3 strong bars on caudal fin, an orange bar at the fin base, a black bar on the fin, and broad white to yellow bar on fin tips; caudal spot weak to absent in young; flank in young with many bronze spots, often forming 8-16 distinct bar = Micropterus dolomieu (Smallmouth Bass/Achigan à petite bouche) [widespread]
Upper jaw extends back to a level behind eye; lateral line scales 58-77 (usually less than 70 in Canada); no scales on the bases of the soft dorsal and anal fin bases; caudal fin in young not clearly barred, yellowish to reddish at base, black distally and only extreme distal margin of fin white; well-developed caudal spot in young; young fish with a flank stripe, sometimes in separate blotches = Micropterus salmoides (Largemouth Bass/Achigan à grande bouche) [widespread]
5. Opercular or "ear" flap black with a red spot at posterior edge (white in preserved fish, rarely absent and flap all black); opercular flap stiff and inflexible at its bony edge; cheeks with wavy blue streaks; gill rakers short and rounded, longest raker not reaching second adjacent raker when appressed (in adults, longer in young but not as long as in young bluegills; length of longest raker less than two times its base width in adults, 3-4 times in young-of-the-year); no black blotch at posterior base of second dorsal fin = *Lepomis gibbosus (Pumpkinseed/Crapet-soleil) [widespread]
Opercular flap all black; flap thin and flexible at its bony edge; head without blue streaks but a blue sheen ventrally; gill rakers relatively long and slender, longest raker reaching base of second or third adjacent raker when appressed (in adults, shorter in young; compare young fish of similar size in these two species; length of longest raker 4-5 times its base width in adults); black blotch at posterior base of second dorsal fin on last 5 rays = *Lepomis macrochirus (Bluegill/Crapet arlequin) [Ottawa and Rideau rivers]
* Note: These two species are known to hybridize freely in Canada, and hybrids breed with parents, giving a continuous range in characters between the two species. Hybridization is not recorded for the NCR but should it occur, identification to species would not be possible.
Key to Percidae (Perches/Perches)
1. Mouth large, upper jaw extending to or beyond eye mid-point; lower edges of preopercle (L-shaped bone in front of operculum or gill cover) serrated; caudal fin forked; branchiostegal rays (bony struts on underside of head) 7-8, usually 7 --> 2
Mouth small, upper jaw not extending beyond anterior quarter of eye margin; lower edges of preopercle smooth; caudal fin rounded or weakly emarginate; branchiostegal rays 5-6, usually 6 --> 4
2. Anal fin soft rays 6-9, usually 7-8; lower jaw teeth about equal in size, no canines; body compressed in cross section; flanks with 5-10 distinctive, wide, dark bars = Perca flavescens (Yellow Perch/Perchaude) [widespread]
Anal fin soft rays 10-14, usually 12-13; lower jaw with a pair of canine teeth at jaw tip; body subcylindrical in cross section; vague, interrupted bars only in young --> 3
3. Adults with large black blotch at rear base of spiny dorsal fin, no other spots on spiny fin membranes; no scales on operculum in adults; 5-8 dark saddles over the back; lower lobe of caudal fin usually white at tip; pyloric caeca 3, about equal in length to stomach = Sander vitreus (Walleye/Doré jaune) [widespread]
Adults without large black blotch at rear base of spiny dorsal fin although membranes with small to moderate half-moon black spots; scales on operculum in adults; 3-4 brown saddles over the back; lower lobe of caudal fin not white at tip; pyloric caeca 3-9, usually 5, shorter than stomach = Sander canadense (Sauger/Doré noir) [Ottawa and Gatineau rivers]
4. Anal fin large, its area about equal to or larger than soft dorsal fin; body elongate and rounded; midline of belly between and behind pelvic fins naked (females), or with enlarged scales (males) --> 5
Anal fin small, its area much less than soft dorsal fin; body more compressed than rounded; midline of belly between and behind pelvic fins scaled or not, but no enlarged scales --> 6
5. First dorsal fin spines 12-17, usually 14-16; lateral scale rows (counted along mid-flank from head to tail) 67-103, usually 77 or more; no deep groove between the snout and lip (premaxillaries not protractile) = Percina caprodes (Logperch/Fouille-roche zébré) [widespread]
First dorsal fin spines 9-13, usually 10-12; lateral scale rows 43-61, usually 52 or less; a deep to weak groove between the snout and lip (premaxillaries protractile) = Percina copelandi (Channel Darter/Fouille-roche Gris) [Quyon stream, Gatineau River]
6. Snout and upper lip separated by a groove (premaxillaries protractile); a single thin anal fin spine --> 7
Snout and upper lip joined, not separated by a groove (premaxillaries not protractile); two anal fin spines, the first thick --> 8
7. Infraorbital (under the eye) and supratemporal (over back of head) head canals not continuous; normally 6 pores or fewer on the infraorbital canal; 1-11, usually 9 or fewer, pores in the preoperculomandibular head canal (canal runs from lower jaw onto the preopercle bone); 9-15, usually 12 or fewer, dorsal fin soft rays; cheek (area below and behind the eye) and nape (area behind the top of the head) usually scaleless or with very few scales; usually 3-4 bars on the caudal fin = *Etheostoma nigrum (Johnny Darter/Raseux-de-terre noir) [widespread]
Infraorbital and supratemporal head canals continuous; normally 8 pores or more on the infraorbital canal; 9-13, usually 11, pores in the preoperculomandibular head canal; 10-17, usually 13 or more, dorsal fin soft rays; cheek and nape usually covered with scales; usually 5-8 bars on the caudal fin = *Etheostoma olmstedi (Tessellated Darter/Raseux-de-terre gris) [widespread]
* Note: Head canals and pores are best seen in specimens injected by capillary action with the temporary stain methylene blue. These two species hybridize and some scientists regard them as a single species (Brett, 1985).
8. Cheeks and opercles scaled (may be obscured by skin); dorsal fin spines longer than eye diameter; soft dorsal fin rays 9-13, usually 10-11 = Etheostoma exile (Iowa Darter/Dard à ventre jaune) [widespread]
Cheeks and opercles not scaled; dorsal fin spines short, about eye diameter or less; soft dorsal fin rays 10-15, usually 12-14 = Etheostoma flabellare (Fantail Darter/Dard barré) [Québec streams]
List of Figures (to come)
1. Pictorial key to families (note body form; number, size, position and shape of fins; presence/absence of barbels; eye size and position).
4. Internal anatomy (from McAllister and Coad, 1985).
5. Teeth arrangement (from Encyclopaedia of Canadian Fish).
6. Scale types (ditto).
8. Lamprey disc.
12. Lower lip broad or narrow (couplet 5 in Catostomidae).
14. External characters of a catfish.
The late Curator of Fishes at the Canadian Museum of Nature, Dr. Don E. McAllister, originated the book version of this work in the early 1970s and offered me the chance to contribute a few species accounts to it, then more, and finally co-authorship as C. G. Gruchy graciously stepped down from the project. Having co-authored a book led to me being offered a post in Iran, hence other parts of this website. Coad (2011a) and Cook et al. (2011) give details of McAllister's contributions to ichthyology.
I am indebted to Dr. Claude B. Renaud, N. Alfonso, M. Picard, Dr. M. Graham and P.
Minns, Canadian Museum of Nature, Ottawa, Anne Phelps and
Brent Campbell, University of Ottawa, and Erling Holm, Royal Ontario Museum,
Toronto for information on fish distributions and for comments on the keys and key
characters. Karen Hamilton, Editor of Trail & Landscape, kindly gave
permission to re-use the identification keys that first appeared in that
journal. Dr. Scott Ercit, Canadian Museum of Nature, Ottawa explained the
mysteries of converting UTMs to latitude/longitude.
Grant Hopkins, Orléans provided copies of articles and newspaper
clippings that added much useful information specific to local captures.
Dr. M. Sawada, Department of Geography, University of Ottawa for advice and
guidance for GIS analyses. Keelan Jacobs served an internship in 2003 at CMN and
helped input mapping data. Suzanne Monette served as an intern in 2002-2003 and
entered a substantial amount of mapping data.
Uta Gruenert, University of Ottawa provided collection data from her field work.
Tim Haxton, Ontario Ministry of Natural Resources took me out sturgeon
fishing on the Ottawa River in 2004, enabling me to garner photographs of this species
and provided copies of various reports on local fishes.
Naomi Langlois-Anderson, South Nation Conservancy, took me trap-netting on
the South Nation River in 2004 enabling me to take photographs of various
species and Amie Boudreau of the same institution provided details on fish
distributions. Ryan Robson took me and ichthyology intern Krystal Lapierre
fishing again on the South Nation in 2005; planning was asssisted by John Irven.
Lisa Setterington, City of Ottawa kindly provided copies of various reports and took me out for a
muddy excursion looking for stranded fishes in the Rideau River system.
Mark McMaster, National Water Research Institute, Burlington kindly sent copies of reports on reproductive function of fishes in
the Ottawa River. Alison Murray showed a photographic foresight that enabled me to add a
variety of images to this work. George Fisher kindly volunteered his expertise
on digital matters during the summer of 2004, improving various aspects of the
website and converting images.
Jonathan Ferrabee, CMN introduced me to an ichthyological desert in the lakes
at high altitude in the Gatineau Hills. Prof. Clifford E. Kraft, Department of
Natural Resources, Cornell University, Ithaca, New York informed me of the
copyright free images from his "Inland Fishes of New York" (http://fish.dnr.cornell.edu/nyfish/fish.html). Studies on this fauna have been in train for almost 50 years and
numerous summer students, interns, volunteers, contractees, staff and members of
the general public have helped in the field, in the laboratory and in letters
and emails to build up collections and knowledge of the fishes. They are too numerous to
mention here but their help has been much appreciated and this work serves as a
tribute to their efforts.
This bibliography covers works on fishes within the National Capital Region of Canada and areas immediately adjacent, and some general overviews of Canadian fishes relevant to local fishes. Scott and Crossman (1973) has a thorough listing of earlier literature and Coker et al. (2001) have some more recent literature. Zoological Record is an ongoing listing of research papers and is available at the Canadian Museum of Nature (at its Pink Road, Gatineau facility). Coverage of newspaper articles does not pretend to be exhaustive but those found are included for their local interest, as records of catches and for mapping distributions. Some clippings were available but lacked dates and/or name of the newspaper - they are not cited here but the information has been used for mapping and in the text, appearing as "newspaper reports".
Sources of information from the web are often cited in the text as an URL, rather than here. The date downloaded is given where known. These links may be broken or only available as cached versions and should be viewed as a documentary source. The following web sites have some information on fishes in the NCR and contain more relevant links and they too may be broken although some effort is made to verify these ones periodically:-
General
www.ontariostewardship.org/ - Links to various organizations concerned with the NCR environment
Sport Fishing
www.mnr.gov.on.ca - Ontario Ministry of Natural Resources (fishing regulations, publications, fish descriptions, etc)
www.fapaq.gouv.qc.ca/ - Société de la faune et des parcs du Québec (fishing regulations, publications, fish descriptions, etc)
www.neatpage.com/fishing/species.htm - Local species
www.rideau-info.com/canal/fishinfo.html - Rideau River fishes
www.muskiescanada.ca - Muskies Canada with Ottawa Chapter
www.ncf.carleton.ca/~ac282/ - Local and larval fishes
www.jeffcyr.com/ - Local fishing including gar
www.ottawafishing.com/index.cfm- Ottawa area fishing
Conservation
www.nation.on.ca/ - South Nation River Conservation
www.mvc.on.ca/ - Mississippi Valley Conservation
www.rvca.ca/ - Rideau Valley Conservation
www.ottawariverkeeper.ca/ - Ecological welfare of the Ottawa River
www.fog-arg.org/ - Friends of the Gatineau River
www.ogwa-hydrog.ca/en/home - Ottawa Gatineau Watershed Atlas
www.canadascapital.gc.ca/places-to-visit/gatineau-park - Gatineau Park
Ichthyology / Biology
www.fishbase.org - Everything about fishes
www.nature.ca/rideau - Canadian Museum of Nature study on the Rideau River
A
Alfonso, N., B. W. Coad and A. R. Gagnon. 2008a. A biogeographic analysis of the ichthyofauna of the National Capital Region. 138th Annual Meeting American Fisheries Society, 17-21 August 2008, Ottawa, Ontario (poster).
Alfonso, N., B. W. Coad and A. R. Gagnon. 2008b. A biogeographic analysis of the ichthyofauna of the National Capital Region. Research Day at Gatineau Park, 18 October 2008 (poster).
Ami, H. M. 1884. List of fossils from Ottawa and vicinity. Ottawa Field-Naturalists' Club Transactions, 5, 2(1):54-62. Anonymous. No date. Gatineau Park. Checklist. Fish/Parc de la Gatineau. Liste des Poissons. National Capital Commission/Commission de la Capitale nationale, Ottawa. 20 pp.
Anderson, M. 2000. The downtown fly fisher Ottawa-Hull. The Canadian Fly Fisher, winter 2000, p. 10-14.
Anonymous. No date. Outaouais, Québec. Fisherman's paradise! Société d'aménagement de l'Outaouais, Québec. 24 pp., map.
Anonymous. 1932. Report of the Ontario Department of Game and Fisheries, 1931. Ontario Department of Game and Fisheries, Toronto.
Anonymous. 1950. South Nation Valley Interim Report 1948. Recommendations and Summary. Fish, pp. 61-64. Department of Planning and Development, King's Printer, Toronto.
Anonymous. 1961a. Close to home. The Ottawa Citizen, 25 April 1961, p. 15.
Anonymous. 1961b. Big Quebec pike. The Ottawa Citizen, 17 July 1961.
Anonymous. 1962a. Pride of Dows Lake. The Ottawa Citizen, 24 April 1962.
Anonymous. 1962b. Ottawa River walleye. The Ottawa Citizen, 27 July 1962.
Anonymous. 1964. Boys beach huge fish. The Ottawa Citizen, 21 August 1964, p. 42.
Anonymous. 1965. Pickerel run reaches peak. The Ottawa Citizen, 27 April 1965.
Anonymous. 1966. Come again, to see pickerel. The Ottawa Citizen, 18 April 1966, p. 16.
Anonymous. 1967. Big pickerel run Friday. The Ottawa Citizen, 20 April 1967.
Anonymous. 1971. 1970-71 winter fishing report, Kemptville District. Ontario Department of Lands and Forests, Kemptville, Ontario.
Anonymous. 1972. 1971-72 winter fishing report, Kemptville District. Ontario Department of Lands and Forests, Kemptville, Ontario. 6 pp.
Anonymous. 1974a. Drôles de poissons. Le Droit, 11 juillet 1974.
Anonymous. 1974b. The young men and the river. The Ottawa Citizen, 30 August 1974, p. 2.
Anonymous. 1979. Long fight, long fish. The Ottawa Citizen, 29 November 1979, p. 27.
Anonymous. 1977. Documentaire sur le bassin de la rivière des Outaouais. Services de la gestion de l'Environnement, Région du Québec, Pêches et Environnement Canada. 195 pp., annexes.
Anonymous. 1980. La faune du ruisseau de la Brasseries, inventaire et possibilités d'utilisation. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Tourisme, de la Chasse et de la Pêche du Québec, Hull. 28 pp., annexes. (N)
Anonymous. 1983. Description et utilisation des ressources fauniques de la région administrative de l'Outaouais. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Tourisme, de la Chasse et de la Pêche du Québec, Hull. 215 pp. (N)
Anonymous. 1987. Documentaire sur le bassin de la rivière des Outaouais. Services de la gestion de l'Environnement, Région du Québec, Pêches et Environnement Canada. 195 pp., annexes.
Anonymous. 1989a. Etude de la fraye de l'esturgeon jaune dans le secteur Hull-Gatineau. Étude présentée par Consor Inc. au Ministère du Loisir, de la Chasse et de la Pêche, Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Hull. 28 pp.
Anonymous. 1989b. Etude de la fraye de l'esturgeon jaune dans le secteur des rivières Rouge et Petite-Nation. Étude présentée par Consor Inc. au Ministère du Loisir, de la Chasse et de la Pêche, Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Hull. 16 pp.
Anonymous. 1992. Liste des espèces de poissons de la rivière des Outaouais dans la région de l'Outaouais. Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull. 6 pp.
Anonymous. 1994. Amazing discovery in Carleton's groundwater project. This Week at Carleton (Carleton University, Ottawa, 31 March 1994), 15(12):1.
Anonymous. 1995. Etude des répercussions environnementals. Central hydroélectrique de Rapides Deschênes, rivière des Outaouais, Le Group-conseil Enviram Inc., rapport présenté à GTM Hydrovolt Inc. 104 pp, annxes.
Anonymous 1998. Sex and the single sucker. Faits saillants/Just the Facts, Canadian Museum of Nature, Museé canadien de la Nature, Aylmer, 5(5):1 p.
Anonymous. 1999a. Sportfishing in Québec. Main Regulations April 1, 1999 - March 31, 2000. Faune et Parcs, Québec. 96 pp.
Anonymous 1999b. Sexy suckers sequel. Faits saillants/Just the Facts, Canadian Museum of Nature, Museé canadien de la Nature, Aylmer, 6(18):1 p.
Anonymous. 1999c. Claude gets an oscar. Faits saillants/Just the Facts, Canadian Museum of Nature/Musée canadian de la Nature, Ottawa, 6(25) (12 July/Juillet 1999): 1 p.
Anonymous. 2001. Rapport de pêche 2000-2001. Observation scientifique des poissons de la rivière des Outaouais. Mini-cours d'enrichissement-sciences, Faculté d'éducation, Université d'Ottawa, Ottawa. 46 pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Anonymous. 2002. OPINION 1991 (Case 3131). Hybognathus stramineus Cope, 1865 (currently Notropis stramineus; Osteichthyes,Cypriniformes): specific name conserved. Bulletin of Zoological Nomenclature, 59(1):58-59.
Appleton, R. 1968. We're killing our river. The Ottawa Citizen, 15 January 1968, p. 2 (supplement).
Arnprior Fish and Game Club. 1984. Walleye creel survey - Madawaska River below Arnprior G.S. 12 May 1984.
Arnprior Fish and Game Club. 1992. Creel survey for Arnprior Headpond, raw data. Unpublished manuscript.
Arsenault, M. 1979. Étude morphologique de poissons fossiles (Mallotus villosus) de la mer Champlain (Pleistocène Supérieur, en provenance de Green Creek, Ottawa: quelques aspects écologiques. Thèse de maîtrise, Université du Québec à Montréal, Québec. 140 pp.
Aubry, P. 1999. Une barbue albinos capturée à Treadwell. Vision Prescott-Russell, 16 juillet 1999.
B
Bailey, R. M. and G. R. Smith. 1981. Origin and geography of the fish fauna of the Laurentian Great Lakes basin. Canadian Journal of Fisheries and Aquatic Sciences, 38(12):1539-1561.
Baldwin, R. G. 1971. 1970 summer fishing report, Kemptville District. Ontario Department of Lands and Forests, Kemptville, Ontario. 5 pp.
Baldwin, R. G. 1971. 1971 summer creel census report for Kemptville District. Ontario Department of Lands and Forests, Kemptville, Ontario.6 pp.
Barker, J. 1999. Fisherman wins Oscar with Amazonian catch. The Ottawa Citizen, 15 July 1999, p. D3.
Beaulieu, M.A. 1977. Rapport sur la répartition géographique des principales espèces de poissons de pêche sportive dans le district de l'Outaouais. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Tourisme, de la Chasse et de la Pêche du Québec, Hull. 27 pp. (N)
Beaulieu, M.-R, S. U. Qadri and J. M. Hanson. 1979. Age, growth, and food habits of the pumpkinseed sunfish, Lepomis gibbosus (Linnaeus), in Lac Vert, Québec. Le Naturaliste canadien, 106(5-6):547-553.
Beebee, E. 2004. Pathfinders. The Guides of the Rideau. The Friends of the Rideau, Smiths Falls, Ontario. 168 pp.
Beebee, E. 2007. Fish Tales. The lure and Lore of the Rideau. Ed Beebee & The Friends of the Rideau. Smiths Falls, Ontario. vi + 266 pp.
Belanger, M., D. Chapleau, P. Gagnon et J. M. Lafrance. 1972. Travaux et observations effectués par le Service de la Faune au printemps 1972. Direction régional de l'Outaouias, Service de l'ménagement et de l'exploitation de la faune, Ministère du Tourisme, de la Chasse et de la Pêche du Québec. 45 pp. (N)
Belisle, T. 1999. Osteological deformities in smallmouth bass (Micropterus dolomieu) from the Rideau and Mississippi Rivers. B.Sc. Honours Thesis, Department of Biology, University of Ottawa. 20 pp., 6 tables, 9 figures
Bendig, A. 1998. Rideau River fisheries assessment report (index netting, nursery habitat inventory, contaminant and fall drawdown monitoring) 1995-1997. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton, Ottawa. viii + 62 pp. (see also under RMOC).
Bendig, A. 1999. 1998 Rideau River fisheries assessment report. Index netting, nursery habitat inventory, contaminant and fall drawdown monitoring. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton, Ottawa. v + 67 pp. (see also under RMOC).
Bergeron, J. F. et J. Brousseau. 1981. Guide des poissons d'eau douce du Québec. Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Gouvernement du Québec. xvii + 217 pp.
Bergeron, J. F. et J. Brousseau. 1982. Guide des poissons d'eau douce du Québec. Direction générale de la Faune, Ministère du Loisir, de la Chasse et de la Pêche, Québec. xxvi + 240 pp.
Bergeron, J. F. et B. Vincent. 1977. Relevés physico-chimiques et biologiques effectués au lac des Écorces, comté de Labelle, canton de Campbell. Direction générale de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Rapport, 10:13-52.
Bergeron, M. 2005. Pour la protection de nos plans d'eau. Pontiac Journal du Pontiac, 20 pril 2005.
Bernatchez, L. et M. Giroux. 1991. Guide des poissons d'eau douce du Québec. Broquet, La Prairie, Québec. xxiv + 304 pp.
Billington, C. 2002. Prescription for a healthy Jock. Part 3: I. Ecology. II. Sustainable land use Trail & Landscape, 36(4):145-149.
Blais, J.-P. et V. Legendre. 1978. Tentatives de création d'eaux à ouananiches: les introductions des saumons atlantiques, Salmo salar, marins et dulcicoles dans les eaux douces du Québec, 1867-1977. Rapport Technique, Service de l'Aménagement et de l'Exploitation de la Faune, Québec. 124 pp.
Bonenfant, A.-M. 1999. Pêche miraculeuse dans le canal Rideau. LeDroit, Ottawa-Hull, 15 Juillet 1999, p. 2.
Boucher, J. 2005. Rapport sur la situation de la barbotte des rapides (Noturus flavus) au Québec. Direction du développement de la fauna, Ministère des Ressources naturelles et de la Fauna du Québec, Secteur Faune Québec. v + 31 pp.
Boucher, J., P. Bérubé and R. Clouthier. 2009. Comparison of the channel darter (Percina copelandi) summer habitat in two rivers from eastern Canada. Journal of Freshwater Ecology, 24(1):19-28.
Boutz, R. and S. Smithers. 1998. South Nation River Fish Population Draft Report. South Nation Conservation and Ontario Ministry of Natural Resources, Kemptville.
Bower, V. 1963. Rod and gun. The Ottawa Citizen, 10 October 1963, p. 22.
Bower, V. 1964. Rod and gun. The Ottawa Citizen, 2 January 1964, p. 24.
Bower, V. 1964. Fish and Game Club winners are announced. The Ottawa Citizen, 22 January 1964, p. 24.
Bowman, J. 2002. Atlas of lake herring waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 70 pp.
Boyle, R. 1977. Age, growth, development, food, fecundity, and maturation of the pumpkinseed, Lepomis gibbosus, in the Ottawa River, near Ottawa and Hull, Canada. B.Sc. Honours Thesis, University of Ottawa, Ottawa, Ontario. 105 pp. (O)
Breton, L. 1978. Compte rendu des visites de frayères de grand brochet (Esox lucius) et des pêches expérimentales aux brochetons à la baie Noire ouest et au marais de Thurso aux printemps 1977 et 1978. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. v + 18 pp.
Brett, B. L. H. 1985. Colonization and gene-flow in the darter Etheostoma nigrum. Program of the 65th Annual Meeting of the American Society of Ichthyologists and Herpetologists, p. 45 (abstract).
Bridges, C. D. B. and C. E. Delisle, 1974. Brief observations concerning the visual pigments of some selected fishes from Lake Heney, Québec, a relict of glacial Lake Gatineau. Vision Research,14:187-193.
Bridges, C. D. B. and C. E. Delisle. 1974. Postglacial evolution of the visual pigments of the smelt, Osmerus eperlanus mordax. Vision Research, 14:345-356.
Brisson, J. D., L. Gauthier, D. Banville, N. Desrosiers et J. Tardiff. 2009. Une nouvelle Liste de la faune vertébrée du Québec. Le Naturaliste canadien, 133(1):48-52.
Brousseau, J. et J. Leclerc. 1976. Clef d'identification des principaux poissons d'eau douce du Québec. Service de l'Aménagement de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec. 80 pp.
Brown, D. 1990. Reel monster hides below Hog's Back. The Ottawa Citizen, 20 June 1990, D1.
Brown, H. M. 1984. Lanark Legacy: Nineteenth century glimpses of an Ontario county. County of Lanark, Perth, Ontario. xiv + 290 pp.
Brownstein, M., R. J. Norstrom and D. W. Peter. 1974. Chemical analysis, 1972-1973. Report 19B:1-22. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 2, Ottawa River Project. University of Ottawa-Division of Biological Sciences, National Research Council of Canada, Ottawa, Ontario. v + 602 pp.
Brunet, R. 1982. Diagnose écologique de 15 lacs du parc de la Gatineau en 1981. Natural Resources Section, National Capital Commission, Ottawa. Unpublished report.
Brunton, D. F. 1998. Nature and Natural Areas in Canada's Capital. An Introductory Guide for the Ottawa-Hull Area. The Ottawa Citizen, Ottawa. 208 pp.
Buie, J. 1998. A big fish doesn't justify a whopping fish story. The Ottawa Citizen, 22 October 1998, p. B5.
Busque, L. 1998. Rapport de pêche 1997-1998. Rivière des Outaouais région d'Ottawa-Carleton. Réseau d'observation active de la Biosphère. Observations des poissons d'eau douce des écosystèmes Saint-Laurent-Grands-Lacs. Mini-cours d'enrichissement-sciences, Faculté d'éducation, Université d'Ottawa, Ottawa. 20 pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Busque, L., C. Bourgault and others. 1999. Rapport de pêche 1998-1999. Observation scientifique des poissons de la rivière des Outaouais. Mini-cours d'enrichissement-sciences, Faculté d'éducation, Université d'Ottawa, Ottawa. 40 pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Butler, D. 2006. Ancient creatures, crown jewels. The Ottawa Citizen, 12 August 2006, p. E1.
C
Campbell, B. G. 2001. A study of the river redhorse, Moxostoma carinatum (Pisces; Catostomidae), in the tributaries of the Ottwa River, near Canada's National Capital and in a tributary of Lake Ontario, the Grand River, near Cayuga, Ontario. M.Sc. Thesis, Ottawa Carleton Institute of Biology, University of Ottawa, Ottawa, Ontario. viii + 137 pp.
Campeau, P. 1999. Le Réservoir du Poisson Blanc. Info Plein Air, 7(2):16.
Campeau, P. 2002. La rivière di Lièvre. Sentier Chasse-Pêche, 31(9):60-64.
Carroll, S. 2000. Rideau River Fisheries Assessment Report. Index Netting, Nursery Habitat Inventory, Contaminant Analysis and Fall Draw Down Monitoring. 1999. Prepared by the Surface Water Quality Branch, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton. viii + 69 pp. (see also under RMOC).
Casselman, J. M. 2007. Determining minimum ultimate size, setting size limits, and developing trophy standards and indices of comparable size for maintaining quality muskellunge (Esox masquinongy) populations and sports fisheries. Environmental Biology of Fishes, 79(1-2):137-154.
Casselman, J. M., C. J. Robinson and E. J. Crossman. 1999. Growth and ultimate length of muskellunge from Ontario waterbodies. North American Journal of Fisheries Management, 19:271-290.
Chabot, J. 1981a. La période de fraie du touladi dans la région de l'Outaouais. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 13 pp.
Chabot, J. 1981b. La période de fraie du grand brochet dans la région de l'Outaouais. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 17 pp.
Chabot, J. 1981c. Effet de la présence d'un barrage de rétention d'eau en relation avec les populations de touladis et de grands brochets du lac St-Patrice, comté de Pontiac. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 17 pp., 3 annexes.
Chabot, J. et J. Caron. 1996. Les poissons de la rivière des Outaouais, de Rapides-des-Joachims à Carillon. Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère de l'Environnement et de la Faune, Hull. viii + 41 pp., annexe 1.
Chabot, J. et H. Fournier. 1986. Evaluation des impacts sur la faune ichtyenne provoqués par les aménagements réalisés principalement en faveur de la sauvagine au marais de Thurso et recommandations. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 54 pp.
Champagne, D. E., C. R. Harington and D. E. McAllister. 1979. Deepwater sculpin, Myoxocephalus thompsoni (Girard) from a Pleistocene nodule, Green Creek, Ontario, Canada. Canadian Journal of Earth Sciences, 16(8):1621-1628.
Chapleau, D. 1974. Rapport sur les visites de frayères effectuées au printemps 1973. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Gouvernement du Québec. iii + 19 pp.
Chapleau, F. and J. A. Cooper. 1992. Variation in the preoperculomandibular canal of the johnny darter, Etheostoma nigrum, with associated zoogeographical considerations. Canadian Journal of Zoology, 70(12):2315-2321.
Chapleau, F., C. S. Findlay and E. Szenasy. 1997. Impact of piscivorous fish introductions on fish species richness of small lakes in Gatineau Park, Québec. Ecoscience, 4(3):259-268.
Chapleau, F. and G. Pageau. 1985. Morphological differentiation of Etheostoma olmstedi and E. nigrum (Pisces: Percidae) in Canada. Copeia, 1985(4):855-865.
Chatelain, R. 1976. Etude de la reproduction du brochet Esoc lucius dans la frayère Pélissier, Lac McGregor, comté Papineau. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Gouvernement du Québec. ii + 19 pp., 5 tabs., 6 photos.
Chaundy, J. 1987. The threat to the river redhorse. Trail & Landscape, 21(2):82-83.
Cholmondeley, R. 1985. 1985 Ottawa River index netting study. Ontario Ministry of Natural Resources, Cornwall, Ontario. 63 pp.
Christie, A. E. 1979. The calculation of temperature guidelines to protect fish resident in the Ottawa River. Ontario Hydro Design and Development Division Report No. 79382.
Christie, A. E. 1981. The calculation of temperature guidelines to protect fish resident in the Ottawa River: a predictive model. Verhandlungen internationale Vereinigung für theoretische und angewandte Limnologie, 21(2):1238-1243.
City of Ottawa. 2002. Rideau River fisheries assessment report Eccolands Reach 2002. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Water Environment Protection Program, Environmental Programs & Technical Support Division, Utility Services Branch, City of Ottawa. v + 57 pp. (authored by L. Setterington).
City of Ottawa. 2004. Rideau River fisheries assessment summary report 1995-2000. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Water Environment Protection Program, Environmental Programs & Technical Support Division, Utility Services Branch, City of Ottawa. vi + 84 pp. (authored by L. Setterington).
Clarke, R. McV. 1973. The systematics of ciscoes (Coregonidae) in central Canada. Ph.D. Thesis, University of Manitoba, Winnipeg, Manitoba. xvii + 219 pp.
Coad, B. W. 1974. Vertebral frequencies with notes on anomalies in samples of threespine sticklebacks (Gasterosteus aculeatus L.) from eastern North America. Canadian Field-Naturalist, 88(2):220-223.
Coad, B. W. 1983a. Plate morphs in freshwater samples of, Gasterosteus aculeatus from Arctic and Atlantic Canada: Complementary comments on a recent contribution. Canadian Journal of Zoology, 61(5):1174-1177.
Coad, B. W. 1983b. The alewife, Alosa pseudoharengus (Wilson) a fish new to the National Capital Region (Osteichthyes: Clupeidae). Trail & Landscape, 17(5):256-258.
Coad, B. W. 1985a. Out damned spot! Trail & Landscape, 19(2):76-77.
Coad, B. W. 1985b. Plate morphs of the threespine stickleback in the National Capital Region. Trail & Landscape, 19(4):220-225.
Coad, B. W. 1986a. The margined madtom, Noturus insignis, in Canada. Trail & Landscape, 20(3):102-108.
Coad, B. W. 1986b. Checklist of the fishes of the Ottawa District. Trail & Landscape, 21(1)(1987):40-60.
Coad, B. W. 1987a. The sex life of the male fathead. Trail & Landscape, 21(2):84-86.
Coad, B. W. 1987b. The spotfin shiner in the Ottawa District. Trail & Landscape, 21(3):141-142.
Coad, B. W. 1987c. Absent records of fishes in the Ottawa District. Trail & Landscape, 21(5):249-254.
Coad, B. W. 1988. Sucker run. Trail & Landscape, 22(2):66-68.
Coad, B. W. 1994. Modelling biodiversity. Science Forum Scientifique, Canadian Museum of Nature/Musée canadien de la nature, November 21 novembre 1994, p. 37 (abstract).
Coad, B. W. 1995. Review of "Lake, River and Sea-Run Fishes of Canada" By Frederick H. Wooding. 1994. Harbour Publishing, Madeira Park, British Columbia. $32.95. Canadian Field-Naturalist, 109(2):282.
Coad, B. W. 2001. Keys to the Fishes of the National Capital Region. Trail & Landscape, 35(3):133-166.
Coad, B. W. 2004. Fishes of Canada: An Annotated Checklist. First posted 4 September 2002 at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Pure Throttle Technologies Inc., 1113, Cameo Drive, Ottawa, Ontario K2C 1Y6.
Coad, B. W. 2007. Modelling Biodiversity of Fishes in Canada's National Capital Region. XII European Congress of Ichthyology, Cavtat (Dubrovnik), Croatia, 9-13 September 2007 (poster and abstract).
Coad, B. W. 2011a. Bibliography of Donald Evan McAllister. Canadian Field-Naturalist, 124(4)(2010):336-356 (http://www.ofnc.ca/cfn/124-4/06_02104_CoadWEB.pdf).
Coad, B. W. 2011b. Biodiversity of fishes in Canada’s National Capital Region. Acta Ichthyologica et Piscatoria, 41(2):89-94 (http://www.aiep.pl/volumes/2010/2_2/pdf/03_949_FULLTEXT.pdf and http://www.fishes.com/Download/index.htm).
Coad, B. W. and N. Alfonso. 2005. The hornyhead chub, Nocomis biguttatus, a fish new to the National Capital Region (Actinopterygii: Cyprinidae). Trail & Landscape, 39(2):68-72.
Coad, B. W. and D. E. McAllister. 2002. Dictionary of Ichthyology. First posted 4 September 2002 at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Pure Throttle Technologies Inc., 1113, Cameo Drive, Ottawa, Ontario K2C 1Y6.
Coad, B. W., P. J. Rubec and S. U. Qadri. 1974. Deformed vertebral columns in brown bullheads, Ictalurus nebulosus (Lesueur), from the Ottawa River. Canadian Field-Naturalist, 88(2):224-226.
Coad, B. W. with H. Waszczuk and I. Labignan. 1995. Encyclopedia of Canadian Fishes. Museum of Nature, Ottawa and Canadian Sportfishing Productions, Waterdown, Ontario. viii + 928 pp.
Coker, G. A., C. B. Portt and C. K. Minns. 2001. Morphological and ecological characteristics of Canadian freshwater fishes. Canadian Manuscript Report of Fisheries and Aquatic Sciences, 2554:iv + 86 pp.
Comtois, A. 2001. L'utilisation des rapides de la rivière Gatineau par les poissons. Projet de 4e année remis au Dr François Chapleau dans le cadre du cours EVS 4001 Projet de recherche, Université d'Ottawa, Ottawa, Ontario. iii + 60 pp.
Comtois, A., F. Chapleau, C. B. Renaud, H. Fournier, B. Campbell et R. Pariseau. 200t6. Inventaire printanier d'une frayère multispécifique: l'ichtyofaune des rapides de la rivière Gatineau, Québec. Canadian Field-Naturalist, 118(4)(2004):521-529.
Cook, F. R., Coad, B. W., Renaud, C. B., Gruchy, C. G. and Alfonso, N. R. 2011. Donald Evan McAllister, 1934-2001: The growth of ichthyological research at the National Museum of Canada/Canadian Museum of Nature. Canadian Field-Naturalist, 124(4)(2010):330-335, 3 figures (http://www.ofnc.ca/cfn/124-4/05_02103_McAllisterWEB.pdf).
Cook, M. and S. Fisher. 1986. Blakeney weir would kill rare fish: scientist. The Ottawa Citizen, 4 March 1986, p. C3.
Copeman, D. G. 1973. Population diversity in the rainbow smelt, Osmerus eperlanus mordax (Mitchill, 1814) (Salmonoidea: Osmeridae) as revealed by canonical and discriminant function analyses on morphometric, meristic and esterase data. Ph.D. Thesis, Memorial University, St. John's, Newfoundland. 231 pp.
Copeman, D. G. 1977. Population differences in rainbow smelt, Osmerus mordax: multivariate analysis of mensural and meristic data. Journal of the Fisheries Research Board of Canada, 34(8):1220-1229.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2002. Canadian Species at Risk, May 2002. Committee on the Status of Endangered Wildlife in Canada, Ottawa. iii + 34 pp.
Cote, M. 1972. 6 big feet of fighting fish. The Ottawa Citizen, 6 July 1972, p. 1 and 3.
Couillard, C. M. and P. V. Hodson. 1996 Pigmented macrophage aggregates: a toxic response in fish exposed to bleached-kraft mill effluent? Environmental Toxicology and Chemistry, 15(10):1844-1854.
Couillard, C. M., P. V. Hodson, P. Gagnon and J. J. Dodson. 1995. Lesions and parasites in white suckers, Catostomus commersoni, in bleached-kraft pulp mill-contaminated and reference rivers. Environmental Toxicology and Chemistry, 14(6):1051-1060.
Crawford, W. J. 1969. Shirley's Bay (Ottawa River) fishing study. Ontario Department of Lands and Forests, Kemptville, Ontario. 3 pp.
Critchlow, D. P. 2000. Summary of 1999 commercial fishing on the Ottawa River. Ontario Ministry of Natural Resources, Kemptville, Ontario. 7 pp. (O)
Crowder, R. 2002. Mississippi smallies on the fly. Part 2. Lines on the Water, Newsletter of the Ottawa Women Fly Fishers, 2(2):2-3.
Cudmore, B. and N. E. Mandrak. 2005. The Baitfish Primer. Fisheries and Oceans Canada, Burlington and Bait Association of Ontario, Peterborough. 36 pp.
Cuerrier, J.-P. 1983. Adaptation of Atlantic Salmon, Salmo salar, to a restricted freshwater environment. Canadian Field-Naturalist, 97(4):439-442.
Cuerrier, J. P. and M. Dadswell. 1969. Limnology and experimental fishery management studies in Gatineau Park during 1968. MS Report, Limnology Section, Canadian Wildlife Service, Ottawa.
Currie, D. J., P. Dilworth-Christie and F. Chapleau. 1999. Assessing the strength of top-down influences on plankton abundance in unmanipulated lakes. Canadian Journal of Fisheries and Aquatic Sciences, 56(3):427-436.
D
Dadswell, M. J. 1972. Postglacial dispersal of four deepwater fishes on the basis of new distributional records in eastern Ontario and western Quebec. Journal of the Fisheries Research Board of Canada, 29(5):545-553.
Dadswell, M. J. 1973. Distribution, ecology, and post-glacial dispersal of certain crustaceans and fishes in eastern North America. Ph.D. Thesis, Department of Biology, Carleton University, Ottawa, Ontario. x + 172 pp.
Dadswell, M. J. 1974. Distribution, ecology, and postglacial dispersal of certain crustaceans and fishes in eastern North America. Publications in Zoology, National Museums of Canada, 11:xviii + 110 pp.
Dadswell, M. J. 1975. Further new localities for certain coldwater fishes in eastern Ontario and western Quebec. Canadian Field-Naturalist, 89(4):447-450.
Dalgleish, C. 2002. Atlas of black crappie waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 15 pp.
Dawley, R. M. and K. A. Goddard. 1988. Diploid-triploid mosaics among unisexual hybrids of the minnows Phoxinus eos and Phoxinus neogaeus. Evolution, 42(4):649-659.
Dawley, R. M., R. J. Schultz and K. A. Goddard. 1987. Clonal reproduction and polyploidy in unisexual hybrids of Phoxinus eos and Phoxinus neogaeus (Pisces; Cyprinidae). Copeia, 1987(2):275-283.
Dawson, J. W. 1891. Note on a fossil fish and marine worm found in the Pleistocene nodules of Green's Creek on the Ottawa. Canadian Record of Science, 3:287-292.
Dawson, J. W. 1893. The Canadian ice age. William V. Dawson, Montreal, Quebec. 301 pp.
Deachman, B. 2002. The lure of snagging a big one. The Ottawa Citizen, 20 June 2002, p. D3.
DeFreitas, A. S. W. 1974. Mercury dynamics in fish. Report 18:1-29. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 2, Ottawa River Project. University of Ottawa-Division of Biological Sciences, National Research Council of Canada, Ottawa, Ontario. v + 602 pp.
DeFreitas, A. S. W. 1976. Mercury uptake and retention by fish. Chapter 15:1-48. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 3, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
DeFreitas, A. S. W., R. J. Norstrom and A. E. McKinnon. 1977. A growth driven model for pollutant accumulation by fish, with parameter values for methyl mercury and PCB's in a population of yellow perch from the Ottawa River. Chapter 32:1-71. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Final Report Volume 2, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Degner, F., S. J. Cooke and F. Phelan. 2002. A report on the 2001 nearshore community index netting program on the Ottawa River-Fitzroy. Queen's University Biology Station Technical report prepared for the Ontario Ministry of Natural Resources (O).
Delisle, C. 1965a. A study on the mass mortality of the stunted smelt population, Osmerus eperlanus mordax, at Heney Lake, Gatineau Co., Que. Ontario Research Foundation, 19th Technical Sessions, Queen's University, Kingston, Ontario. 15 pp. (mimeo).
Delisle, C. 1965b. Infection des fretins d'éperlans Osmerus eperlanus mordax (Mitchill) par Glugea hertwigi Weissenberg, 1911 au lac Heney, Comté de Gatineau, Québec. Annales de l'ACFAS, 33:74.
Delisle, C. 1969a. Écologie, croissance et comportement de l'éperlan du Lac Heney, Comté de Gatineau ainsi que la répartition en eau douce au Québec. Thèse doctorale, Département de Biologie, Université d'Ottawa, Ontario. 161 pp. + appendice.
Delisle, C. 1969b. Bimonthly progress of a non-lethal infection by Glugea hertwigi in young-of-the-year smelt, Osmerus eperlanus mordax. Canadian Journal of Zoology, 47(5):871-876, plates I-IV.
Delisle, C. and W. Van Vliet. 1968. First records of the sculpins, Myoxocephalus thompsonii and Cottus ricei from the Ottawa Valley, southwestern Quebec. Journal of the Fisheries Research Board of Canada, 25(12):2733-2737.
Delisle, C. et C. Veilleux. 1969. Répartition géographique de l'éperlan arc-en-ciel Osmerus eperlanus mordax et de Glugea hertwigi (Sporozoa, Microsporidia) en eau douce, au Québec. Le Naturaliste canadien, 96(3):337-358.
Department of Lands and Forests, Ontario. 1964. A history of Kemptville. District History Series, Department of Lands and Forests, Ontario, 17:112 pp. (O)
Desroches, J-F. 2009. Proposition de changements de noms français pour quelques espèces de poissons d'eau douce du Québec. Le Naturaliste canadien, 113(1):73-79.
Desroches, J-F., D. Pouliot, L. Picard and R. Laparé. 2008. Nouvelles mentions pour six espèces de poissons d'eau douce rares au Québec. Le Naturaliste canadien, 132(2):62-66.
Dey, R. 1991. Preliminary muskellunge spawning study, Ottawa River (Chenaux to Fitzroy). Project Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario. 11 pp.
Dick, T. A., S. R. Jarvis, C. D. Sawatzky and D. B. Stewart. 2006. The lake sturgeon, Acipenser fulvescens (Chondrostei: Acipenseridae): an annotated bibliography. Canadian Technical Report of Fisheries and Aquatic Sciences, 2671:iv + 251 pp.
Dickenson, D. 2002. South Nation River fish being studied. The Prescott Journal (Online Edition), 172(34): 1 p.
Dimension Environment Ltd. 1977. Natural resource inventory Rideau Canal. Report by Dimension Environment Ltd. for Parks Canada. vii + 94 pp.
Dion, B. 1986. Inventaire des espèces de poissons fréquentant le marais des Laîches et vérification de la mortalité hivernale. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 17 pp., annexes
Dion, B. et R. Blias. 1988. Utilisation pour la pêche sportive et la pêche commerciale du territoire québécois de la rivière des Outaouais en 1985-1986. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 64 pp., annexes.
Dubreuil, R. and J. P. Cuerrier. 1950. Rapport sur l'étude du cycle de maturation des glandes génitales chez l'esturgeon de lac (A. fulvescens Raf.) de la rivière Ottawa, 1949. Ministère d'Industrie et Commerce, Québec et Conseil National de Recherches, Ottawa. Dactylogramme. 63 pp. (OMNR Kemptville)
Dumont, P. 1981. Le contrôle de l'exploitation de l'omble de fontaine dans la région administrative de l'Outaouais. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 27 pp.
Dumont, P. 1982. Dispersion post-glaciaire de l'omble chevalier d'eau douce (Salvelinus alpinus) dans le Québec méridional. Le Naturaliste canadien, 109(2):229-234.
Dumont, P., J. F. Bergeron, P. Dulude, Y. Mailhot, A. Rouleau, G. Ouellet and J.-P. Lebel. 1988. Introduced salmonids: where are they going in Quebec watersheds of the Saint-Laurent River? Fisheries, 13(3):9-17.
Dumont, P. et M. Monette. 1979. Distribution, écologie et statut de l'omble chevalier d'eau douce (Salvelinus alpinus Linnaeus) dans l'Outaouais québécois. District de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 35 pp. (N)
Dunford, W. E. 1977. Aquatic biological studies, Chats Falls GS - 1977. Research Division, Ontario Hydro, Report No. 77-463-K. 65 pp.
Dunford, W. E. 1978. Arnprior Reservoir fish studies, 1977. Ontario Hydro Research Division, Toronto, Report No. 78-176-K. 27 pp.
Duquette, P. 1999. Un poisson tropical pêché dans le canal Rideau. Bonjour Dimanche, 18 Juillet 1999, p. 6.
Dymond, J. R. 1939. The fishes of the Ottawa region. Contributions of the Royal Ontario Museum, 15:1-43.
E
Easton, R. 1968. Sturgeon study - Ottawa River. Ontario Department of Lands and Forest, Kemptville, Ontario. 32 pp.
Egan, K. 1996a. Hawkesbury fisherman hooks rare salmon in Ottawa River. The Ottawa Citizen, 1 November 1999, p. D2.
Egan, K. 1996b. Just one that got away? The Ottawa Citizen, 6 November 1996, p. C3.
Egan, K. 1997. Salmon 'run' continues. The Ottawa Citizen, 30 October 1997, p. D3.
Egan, K. 1999. The Wright stuff. The Ottawa Citizen, 24 October 1999, p. C8-C11.
Egan, K. 2000. Tracking the toothless 'T-Rex' of the Ottawa River. The Ottawa Citizen, 30 August 2000, p. C1, C7.
Egan, K. 2002. Look what's living in the Ottawa River. The Ottawa Citizen, 27 September 2002, p. F1, F6.
Egan, K. 2003. Chronicling the sturgeon's tale. The Ottawa Citizen, 7 June 2003, p. C1.
Ellah, R. T. 1966. Sturgeon tagging on the Ottawa River. Ontario Department of Lands and Forests, Kemptville, Ontario. 3 pp.
Elliot, D. B. 1976. Ottawa and Nation rivers creel census report, winter 1976. Ontario Ministry of Natural Resources, Cornwall, Ontario. 14 pp.
Emmett, B. and P. A. Cochran. 2010. The response of a piscivore (Micropterus salmoides) to a venomous prey species (Noturus gyrinus). Journal of Freshwater Ecology, 25(3):475-479.
Envirocon Ltd and Proctor & Redfern Ltd. 1979. Chats Falls GS B Nuclear Site Baseline Environmental Studies, 1978-1979. Volume 1. Consultants report to Ontario Hydro Environmental Studies and Assessments Department.
Environnement Illimité Inc. 2001. Évaluation de l’habitat du poisson – axe McConnell-Laramée. Rapport présenté au ministère des Transports, Direction de l’Outaouais. 13 pp.
Équipe de Rétablissement du Fouille-Roche Gris. 2001. Plan de rétablissement du fouille-roche gris (Percina copelandi) au Québec. Direction du développement de la faune, Société de la faune et des parcs du Québec. 34 pp.
Eschmeyer, W. N. (Ed.). 1998. Catalog of Fishes. California Academy of Sciences, San Francisco. 3 volumes, CD-ROM.
F
Faber, D. J. (Ed.). 1972. A high school field and laboratory study of Lac Lapêche in Gatineau Park, Québec, during March 1972. Syllogeus, Ottawa, 4:1-25.
Faber, D. J. 1980. Observations on the early life of the golden shiner Notemigonus crysoleucas (Mitchell), in Lac Heney, Québec, p. 69-78. In: Proceedings of the Fourth Annual Larval Fish Conference, 1980. United States Fish and Wildlife Service, Biological Services Program, National Power and Plant Team, Ann Arbor, Michigan.
Faber, D. J. 1981. A light trap to sample littoral and limnetic regions of lakes. Verhandlungen internationale Vereinigung für theoretische und angewandte Limnologie, 21(2):776-781.
Faber, D. J. 1982. Fish larvae caught by a light-trap at littoral sites in Lac Heney, Québec, 1979 and 1980, p. 42-46 In: Bryan, C. F., J. V. Conner and F. M. Truedale (Eds.). Proceedings of the Fifth Annual Larval Fish Conference, Louisiana Cooperative Fisheries Research Unit, Baton Rouge.
Faber, D. J. 1984a. Water babies. Larval fishes of Ottawa and vicinity. Part I. Distribution and phenology of baby fish in lakes and ponds. Trail & Landscape, 18(2):84-92.
Faber, D. J. 1984b. Water babies. Larval fishes of Ottawa and vicinity. Part II. Anatomy of larval fishes. Trail & Landscape, 18(4):198-204.
Faber, D. J. 1984c. Water babies. Larval fishes of Ottawa and vicinity. Part III. Larval fishes of shallow water species. Trail & Landscape, 18(5):273-283.
Faber, D. J. 1985a. Water babies. Larval fishes of Ottawa and vicinity. Part IV. Larval fishes of deep water species. Trail & Landscape, 19(1):18-25.
Faber, D. J. 1985b. The early development of the northern redbelly dace, Phoxinus eos (Cope). Canadian Journal of Zoology, 63(7):1724-1729.
Faber, D. J. 1999. Water babies. Larval fish of Ottawa and vicinity. Ottawa Field-Naturalists' Club, Ottawa. iii + 84-92, 198-204, 273-283, 18-25 (reprint of Faber (1984a, 1984b, 1984c,1985a)).
Fancy, K. 1995. Take a ride on the Rideau. Ontario Fisherman, 15(14):88-93 (Fishing Annual 1995).
Fancy, K. 1996. Parliament Hill musky. Ontario Fisherman, 16(5):21-23.
Ferguson, M. M. and G. A. Duckworth.1997. The status and distribution of lake sturgeon, Acipenser fulvescens, in the Canadian provinces of Manitoba, Ontario and Quebec: a genetic perspective. Environmental Biology of Fishes, 48(1-4):299-309.
Finn, J. 1964. Fishing great in Val-des-Bois area. The Ottawa Citizen, 4 June 1964, p. 18.
Fisher, S. 1987. Conservation officers busy as pickerel run spawns poachers. The Ottawa Citizen, 30 April 1987, p. C3.
Fisheries and Environment Canada. 1977. Monograph on the Ottawa River basin. Inland Waters Directorate, Québec Region, Québec.
Fletcher, J. 1898. Sub-excursions. The Ottawa Naturalist, 12(2):61-63.
Fortin, C., I. Cartier et M. Ouellet. 2005. Rapport sur la situation de la lamproie du nord (Ichthyomyzon fossor) au Québec. Direction du développement de la faune, Ministère des Ressources naturelles et de la Faune, Québec. v + 23 pp.
Fortin, R., S. Guénette, et P. Dumont. 1992. Biologie, exploitation, modélisation et gestion des populations d'esturgeon jaune (Acipenser fulvescens) dans 14 réseaux de lacs et de rivières du Québec. Service de l'aménagement et de l'exploitation de la faune et service de la faune aquatique, Ministère du Loisir, de la Chasse et de la Pêche du Québec. xxi + 213 pp.
Fournier, H. 1988. L'esturgeon jaune de la rivière des Outaouias. Problématique et proposition d'études pour statuer sur sa gestion. Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 11 pp.
Fournier, H., P. Houde et G. Thellen. 1990. Evaluation de la qualité de l'habitat de fraye du grand brochet dans le bief Hull-Carillon. Direction régionale de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère de l'Environnement et de la Faune, Hull, Québec. 16 pp.
Fraser, D., R. Schoenert, R. Thivierge et R. Boutin. 1983. Inventaire ecologiques de lacs du Parc de la Gatineau. Rapport pour la Commission de la Capitale Nationale, Ottawa-Hull, Canada. Eco-Recherches Inc., Doc. 1983. 16.
G
Gaffield, C. 1997. History of the Outaouais. Institut québécois de recherche sur la culture. Les Presses du l'Université Laval, Québec. xvi + 843 pp.
Gagnon, M. M., D. Bussieres, J. J. Dodson and P. V. Hodson. 1995. White sucker (Catostomus commersoni) growth and sexual maturation in pulp mill-contaminated and reference rivers. Environmental Toxicology and Chemistry, 14(2):317-327.
Gardiner, B. G. 1966. Catalogue of Canadian fossil fishes. Royal Ontario Museum, Life Sciences Contribution, 68:1-154.
Garvey, M. 2001. A comparison of spawning lake sturgeon (Acipenser fulvescens) in Lac Deschenes and Lower Allumette. Southcentral Science and Information Section, Ontario M-ministry of Natural Resources, Kemptville. 12 pp.
Gauthier, M. E. 1987. Ottawa River index netting study, Cumberland to Carillon. Ontario Ministry of Natural Resources, Cornwall, Ontario. 61 pp.
Geddes, H. 1992. The Canadian Mississippi River. General Store Publishing House, Burnstown, Ontario. 291 pp.
Gélinas, N., S. Lepage et C. Grondin. 1993. Expérimentation d'une technique de vidange de l'eau d'un marais endigué (marais aux Massetes) en août 1990 afin d'évacuer la communauté ichtyenne restée captive suite au retrait des crues printanières. Entente cadre concernant un plan quinquennal pour la protection et l'aménagement des habitats fauniques. Volet 1.G. Ministère du Loisir, de la Chasse et de la Pêche du Québec et Canards Illimités Canada, Québec. 111 pp.
Goddard, K. A., R. M. Dawley and T. E. Dowling. 1989. Origin and genetic relationships of diploid, triploid, and diploid-triploid mosaic biotypes in the Phoxinus eos-neogaeus unisexual complex, p. 268-280. In: Dawley, R. M. and J. P. Bogart (Eds.). Evolution and Ecology of Unisexual Vertebrates. Bulletin of the New York State Museum, 466:v + 302 pp.
Goodchild, C. D. 1990a. Status of the margined madtom, Noturus insignis, in Canada. Canadian Field-Naturalist, 104(1):29-35.
Goodchild, C. D. 1990b. Status of the brook silverside, Labidesthes sicculus, in Canada. Canadian Field-Naturalist, 104(1):36-44.
Goodchild, C. D. 1994a. Status of the tessellated darter, Etheostoma olmstedi, in Canada. Canadian Field-Naturalist, 107(4)(1993):423-430.
Goodchild, C. D. 1994b. Status of the channel darter, Percina copelandi, in Canada. Canadian Field-Naturalist, 107(4)(1993):431-439.
Grant, R . E. 1996. A review of fish habitat compensation projects in the Grenville/West Dundas area. Technical Report, Ontario Ministry of Natural Resources, Kemptville, Ontario. 148 pp. (N)
Gruchy, C. G. 1968. Two late Quaternary Salmonidae (Pisces) from the Ottawa region, Canada. Vestník Ceskoslovenské Spolecnosti Zoologické (Acta Societatis Zoologicae Bohemoslovacae), 32(4):337-341.
Guay, G. 1981. Recensement de pêche 1980 - parc de la Gatineau. Commission de la Capitale Nationale/National Capital Commisssion, Ottawa. 94 pp.
Guénette, S., R. Fortin and E. Rassart. 1993. Mitochondrial DNA variation in lake sturgeon (Acipenser fulvescens) from the St. Lawrence River and James Bay drainage basins in Quebec, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 50(3):659-664.
Guitard, A., E. Sévigny and D. Mugemana. 2003. Construction of McConnell-Laramée Boulevard between Highway 50 and Chemin de la Montagne. Canadian Environmental Assessment Act Screening Report prepared by Transport Canada, Fisheries and Oceans Canada and the National Capital Commission. vii + 66 pp. ( www.tc.gc.ca/programs/surface/highways/project/doc/screening%20Report%20McConnell-Laramee.pdf, downloaded 23 June 2004)
Gunn, J. 1976. A study of the foraging activities of the brown bullhead Ictalurus nebulosus in the Ottawa River, near Ottawa and Gatineau. Chapter 10:1-14. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 3, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Gunn, J. 1976. The diet, assimilation efficiency of algae and body composition of brown bullhead Ictalurus nebulosus in the Ottawa River, near Ottawa and Gatineau. Chapter 11:1-17. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 3, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Gunn, J. M. 1975. A study of the foraging activities of the brown bullhead, Ictalurus nebulosus, in the Ottawa River, near Ottawa and Gatineau. In: Distribution and transport of pollutants in flowing water ecosystems. Annual Report No. 3, Ottawa River Project. University of Ottawa-Division of Biological Sciences National Research Council of Canada, Ottawa, Ontario. Chapter 10:1-16. (C)
Gunn, J. M. 1976. Algae as an energy source for the omnivorous brown bullhead, Ictalurus nebulosus (Le Sueur) in the Ottawa River. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. iv + 88 pp. (O)
Gunn, J. M., S. U. Qadri and D. C. Mortimer.1977. Filamentous algae as a food source for the brown bullhead (Ictalurus nebulosus). Journal of the Fisheries Research Board of Canada, 34(3):396-401.
H
Haglund, T. R., D. G. Buth and R. Lawson. 1992. Allozyme variation and phylogenetic relationships of Asian, North American, and European populations of the ninespine stickleback, Pungitius pungitius, pp. 438-452. In: Mayden, R. L. (Ed.). Systematics, Historical Ecology, and North American Freshwater Fishes. Stanford University Press, Stanford, California. xxvi + 969 pp.
Halkett, A. 1906. Report of the Canadian Fisheries Museum. 38th Annual Report of the Department of Marine and Fisheries, Appendix, 14:362-370.
Halkett, A. 1908. Report of the Canadian Fisheries Museum. 40th Annual Report of the Department of Marine and Fisheries, Appendix, 14:321-349.
Halkett, A. 1913a. Check List of the Fishes of the Dominion of Canada and Newfoundland. King's Printer, Ottawa. 138 pp.
Halkett, A. 1913b. Excursions. Stittsville. The Ottawa Naturalist, 27(5-6):74-75.
Halliday, J. 1985. Winter kill of fishes in Mud Lake. Trail & Landscape, 19(2):75.
Hand, E. 2007. Fish and the city. Ottawa, Ontario, p. 42-43. Outdoor Canada, Fishing, 35(1):34-52.
Hanson, J. M. 1980. Growth and ecological interactions of the young-of-the-year of three species of sunfish (Centrarchidae) in the Ottawa River near Ottawa, Canada. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. xx + 229 pp.
Hanson, J. M. and S. U. Qadri. 1979. Seasonal growth, food, and feeding habits of young-of-the-year black crappie in the Ottawa River. Canadian Field-Naturalist, 93(3):232-238.
Hanson, J. M. and S. U. Qadri. 1980a. Observations on the biology of the black crappie, Pomoxis nigromaculatus (Le Sueur), in the Ottawa River. Le Naturaliste canadien, 107(1):35-42.
Hanson, J. M. and S. U. Qadri. 1980b. Morphology and diet of young-of-the-year burbot, Lota lota, in the Ottawa River. Canadian Field-Naturalist, 94(3):311-314.
Hanson, J. M. and S. U. Qadri. 1984. Feeding ecology of age 0 pumpkinseed (Lepomis gibbosus) and black crappie (Pomoxis nigromaculatus) in the Ottawa River. Canadian Journal of Zoology, 62(4):613-621.
Harington, C. R. 1971. The Champlain Sea and its vertebrate fauna. Part I. The history and environment of the Champlain Sea. Trail & Landscape, 5(5):137-141.
Harington, C. R. 1972. The Champlain Sea and its vertebrate fauna. Part II. Vertebrates of the Champlain Sea. Trail & Landscape, 6(1):33-39.
Harington, C. R. 1978. Quaternary vertebrate faunas of Canada and Alaska and their suggested chronological sequence. Syllogeus, Ottawa,15:1-105.
Harington, C. R. 1983. Significance of the fossil locality at Green Creek, Ontario. Trail & Landscape, 17(3):164-178.
Harington, C. R. 2003. Quaternary vertebrates of Québec: a summary. Géographie physique et Quaternaire, 57(1):85-94.
Harkness, W. J. K. and J. R. Dymond. 1961. The lake sturgeon: the history of its fishery and problems of conservation. Fish and Wildlife Branch, Ontario Department of Lands and Forests, Toronto. 121 pp.
Hart, M. 1987. Rideau River fisheries population and angler effort and harvest summaries for 1984 and 1985. Ontario Ministry of Natural Resources, unpublished report.
Hart, M. L. 1973a. 1973 Ottawa River creel census. Ontario Ministry of Natural Resources, Kemptville, Ontario. 15 pp.
Hart, M. L. 1973b. Technical upgrading and gear development of the Ottawa River commercial fishery, Cornwall District. Fisheries Industrial Development Project 602-73, Ontario Ministry of Natural Resources, Cornwall, Ontario. 9 pp.
Haxton, T. 1997. A review of the early season walleye harvest from the Ottawa River. Ontario Ministry of Natural Resources, Pembroke, Ontario. 16 pp.
Haxton, T. 1998. Synthesis of fisheries data from the Upper Ottawa River (Mattawa to Arnprior). Ontario Ministry of Natural Resources, Pembroke, Ontario. 30 pp.
Haxton, T. 1999. Nearshore community index netting of Lac des Chats (Ottawa River). Ontario Ministry of Natural Resources, Pembroke, Ontario. 19 pp.
Haxton, T. 2000. Progress report on the tagging of walleye from various spawning populations in the Upper Ottawa River. Ontario Ministry of Natural Resources, Kemptville, Ontario. 17 pp.
Haxton, T. 2002. An assessment of lake sturgeon (Acipenser fulvescens) in various reaches of the Ottawa River. Journal of Applied Ichthyology, 18(4-6):449-454.
Haxton, T. 2003. Fisheries inventory in the channel between Fitzroy Provincial Park and Helen Island, Ottawa River. Ontario Ministry of Natural Resources, Kemptville, Ontario. 7 pp.
Haxton, T. J. 2006. Characteristics of a lake sturgeon spawning population sampled a half century apart. Journal of Great Lakes Research, 32:124-130
Haxton, T. J. 2007. Impacts of water power management on select fish in the Ottawa River, Canada, with an emphasis on lake sturgeon. Ph.D. Thesis, University of Ottawa, Ottawa.
Haxton, T. 2008. A synoptic review of the history and our knowledge of lake sturgeon in the Ottawa River. Southern Science and Information Technical Report, Ontario Ministry of Natural Resources, Peterborough, SSI #126:vi + 31 pp.
Haxton, T. and D. Chubbock. 2002. Review of the historical and existing natural environment and resource uses on the Ottawa River. Southcentral Science and Information Section, Science and Information Branch, Ontario Ministry of Natural Resources, North Bay, Technical Report, 119:viii + 76 pp.
Haxton, T. J. and F. S. Findlay. 2008. Variation in lake sturgeon (Acipenser fulvescens) abundance and growth among river reaches in a large regulated river. Canadian Journal of Fisheries and Aquatic Sciences, 65:645-657.
Haxton, T. and K. Punt. 2000. Walleye telemetry study to determine the spawning locations in the Lac des Chats and Allumette Lake sections of the Ottawa River. Ontario Ministry of Natural Resources, Kemptville and Pembroke, Ontario. 28 pp., appendcies A-V.
Haxton, T. and K. Punt. 2004. Abundance and demography of channel catfish in a large northern river, North American Journal of Fisheries Management, 24:1440-1446.
Hénault, M. 1986. Statut taxonomique et bio-écologie de la population de cisco de lac (Coregonus artedii) frayant le printemps au lac des Écorces, QC. Mémoire de maîtrise, Université du Québec à Montréal, Québec. 160 pp.
Hénault, M. 1987. Statut taxonomique et bio-écologie de la population de cisco de lac (Coregonus artedii) frayant le printemps au lac des Écorces, QC. Direction de la faune aquatique, Service des espèces d'eau fraîche, Ministère du Loisir, de la Chasse et de la Pêche, Québec, Rapport technique, 87-01:xiv + 164 pp.
Hénault, M. et R. Fortin. 1986. Etude comparative des phenotypes morphologiques de stocks de ciscos de lac (Coregonus artedii) frayant au printemps et a l'automne dans la region de Mont-Laurier, Quebec. Canadian Committee for Freshwater Fisheries Research, Ottawa, 4-5 January 1986 (résumé).
Hénault, M. and R. Fortin. 1989. Comparison of meristic and morphometric characters among spring- and fall-spawning ecotypes of cisco (Coregonus artedii) in southern Quebec, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 46(1):166-173.
Hénault, M. and R. Fortin. 1990. Status report on the spring cisco Coregonus artedi ssp. in Canada. Committee on the Status of Endangered Wildlife in Canada (COSEWIC), Canadian Wildlife Service, Ottawa, 13 pp., 3 figs.
Hénault, M. and R. Fortin. 1991. Early life stages, growth, and reproduction of spring-spawning ciscoes (Coregonus artedii) in Lac des Écorces, Quebec. Canadian Journal of Zoology, 69(6):1644-1652.
Hénault, M. et R. Fortin. 1993. Une population unique au Canada: les ciscos de lac (Coregonus artedi) frayant au printemps dans le Lac des Écorces, Quebec. Polskie Archiwum Hydrobiologii, 39(3-4):317-324.
Hénault, M. et R. Fortin. 1994. Statut de la population de cisco de printemps, Coregonus sp., au lac des Écorces, Québec, Canada. Canadian Field-Naturalist, 107(4)(1993):402-409.
Hendrick, A. 1991. 1989 Ottawa River index netting survey. Ontario Ministry of Natural Resources, Cornwall, Ontario. 36 pp.
Hopkins, G. 1987. At 94, Harry Sweeney still fishes Ottawa River. The Ottawa Citizen, 27 August 1987, p. C10.
Hopkins, G. 1989. Homely gar offers unique challenge. The Ottawa Citizen, 13 July 1989, p. B9.
Hopkins, G. 1989. Dead muskellunge a worry for local support group. The Ottawa Citizen, 17 August 1989, p. D6.
Hopkins, G. 1990a. Trout stream may be lost. The Ottawa Citizen, 1 March 1990, p. C7.
Hopkins, G. 1990b. Sheepshead becoming a more common catch. The Ottawa Citizen, 23 August 1990, p. E7.
Hopkins, G. 1990c. Feared lamprey eels still lurking in area river. The Ottawa Citizen, 27 September 1990, p. B7.
Hopkins, G. 1991a. Fishing at your doorstep an attractive change. The Ottawa Citizen, 9 June 1991, p. C6.
Hopkins, G. 1991b. Catching brown trout no tall tale. The Ottawa Citizen, 21 July 1991, p. D2.
Hopkins, G. 1991c. Bridge threatens Rideau fish habitat. The Ottawa Citizen, 29 September 1991, p. D6.
Hopkins, G. 1991d. Ministry yet to solve puzzle of deformities in smallmouth. The Ottawa Citizen, 6 October 1991, p. D7.
Hopkins, G. 1991e. Good news, bad news net result of area river surveys. The Ottawa Citizen, 10 November 1991, p. C6.
Hopkins, G. 1993a. Mystery of the missing brown trout. The Ottawa Citizen, 16 May 1993, p. F5.
Hopkins, G. 1993b. Kanata fishing hole popular with anglers. The Ottawa Citizen, 1 August 1993, p. E6.
Hopkins, G. 1993c. Counting fish helps protect sensibilities of Poole Creek. The Ottawa Citizen, 5 September 1993, p. C8.
Hopkins, G. 1993d. Natural resources ministry may introduce brown trout into local streams. The Ottawa Citizen, 5 December 1993, p. C6.
Hopkins, G. 1994. Palladium may help bring fishing back to ailing Carp River. The Ottawa Citizen, 13 February 1994, p. B5.
Hopkins, G. 1995. Wintertime quest for walleye seems to make spring come a little faster for anglers. The Ottawa Citizen, 24 December 1995, p. B5.
Hopkins, G. 1996a. Debate rages somewhere over the rainbow. The Ottawa Citizen, 1 February 1996, p. C4.
Hopkins, G. 1996b. Top 10 fishing holes. The Ottawa Citizen, 20 June 1996, p. D5.
Hopkins, G. 1997a. Disagreement delays trout stocking plan. The Ottawa Citizen, 15 May 1997, p. E5.
Hopkins, G. 1997b. Top 10 places to fish in the city. The Ottawa Citizen, 3 July 1997, p. B3.
Hopkins, G. 1997c. Club brings brown trout back to Ottawa. The Ottawa Citizen, 17 July 1997, p. B7.
Hopkins, G. 1997d. Survey shows Rideau's fish varied in city. The Ottawa Citizen, 21 August 1997, p. B6.
Hopkins, G. 2000. Enhancement of fishing opportunities in the Ottawa area. Report to the Ontario Ministry of Natural Resources, Kemptville, Ontario. iii + 46 pp.
Hopkins, G. 2005. Eastern-Ontario sturgeon mysteries. Ontario Out of Doors, 37(2):14.
Hopkins, G. 2006. Fishing the lower Ottawa River. Ontario Out of Doors, 38(3):16-25.
Houde, P. 1987. Estimation de l'effort de pêche sur la rivière des Outaouais du barrage de Quyon à la rivière Rouge au printemps 1987. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 22 pp.
Houde, P et H. Fournier. 1992. Travaux de recherches de frayères dans la rivière des Outaouais et ses principaux affluents au cours du printemps 1989. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 82 pp., annexes.
Houston, J. 1987. Status of the lake sturgeon, Acipenser fulvescens, in Canada. Canadian Field-Naturalist, 101(2):171-185.
Houston, J. 1990. Status of the banded killifish, Fundulus diaphanus, in Canada. Canadian Field-Naturalist, 104(1):45-52.
Houston, J. 1996a. The status of the blackchin shiner, Notropis heterodon, in Canada. Canadian Field-Naturalist, 110(3):483-488.
Houston, J. 1996b. The status of the rosyface shiner, Notropis rubellus, in Canada. Canadian Field-Naturalist, 110(3):489-494.
Hubbs, C. L. and K. F. Lagler. 1964. Fishes of the Great Lakes Region. The University of Michigan Press, Ann Arbor. xv + 213 pp.
Hubbs, C. L., K. F. Lagler and G. R. Smith. 2004. Fishes of the Great Lakes Region Revised Edition. The University of Michigan Press, Ann Arbor. xvii + 276 pp., pls. 1-32.
Hutchinson, B. 1993. Natural Resource Conservation Rideau Canal: A Strategic Decision Analysis. Parks Canada, Smiths Falls and Cornwall, Ontario. 44 pp.
Hydro-Québec. 1996. Caracterisation de l'habitat du maskinonge dans le reservoir Carillon entre Thurso et Fasset. Rapport final présenté à Hydro-Québec par Département des sciences biologiques, Université du Québec à Montréal. xvii + 183 pp.
J
Jessiman, B. 1986. Selection of ecological reserves (lakes) in Gatineau Park. Resources Conservation Section, National Capital Commission, Ottawa. Unpublished report.
Joswiak, G. R., R. H. Stasiak and B. F. Koop. 1985. Diploidy and triploidy in the hybrid minnow, Phoxinus eos x Phoxinus neogaeus (Pisces: Cyprinidae). Experientia, 41(4):505-507.
K
Kerr, S. J. 1992. A summary of 1991 muskellunge angler diary information for selected eastern Ontario waters. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Kemptville, Ontario. 7 pp.
Kerr, S. J. 1993. Results from the 1992 cooperative muskellunge angler diary program in eastern Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Kemptville, Ontario. 18 pp.
Kerr, S. J. 1996. A summary of muskies Canada Inc. angler log information, 1979-1994. Science and Technology Transfer Unit, Technical Report TR-011, Ontario Ministry of Natural Resources, Kemptville, Ontario. 107 pp. (N)
Kerr, S. J. 1997. The Fishery of Bennett Lake. Science and Technology Transfer Unit Technical Report TR-012, Ontario Ministry of Natural Resources, Kemptville, Ontario. iii + 36 pp., 4 appendices.
Kerr, S. J. 1999a. A survey of twelve winter fisheries in Lanark County during the winter of 1998-99. File Report, Southcentral Sciences Section, Ontario Ministry of Natural Resources, Kemptville, Ontario. 12 pp. + appendices.
Kerr, S. J. 1999b. A survey of competitive fishing events in Ontario. Southcentral Sciences Section Technical Report TR-114, Ontario Ministry of Natural Resources, Kemptville, Ontario. i + 11 pp. + appendix.
Kerr, S. J. 1999c. Mississippi Lake and its fishery. Southcentral Sciences Section Technical Report TR-115, Ontario Ministry of Natural Resources, Kemptville, Ontario. ii + 36 pp. + 5 appendices.
Kerr, S. J. (Ed.). 1999d. Competitive fishing in Ontario. Workshop Proceedings March 12-13, 1999, Kemptville, Ontario. Workshop Proceedings WP-01, Southcentral Sciences Section, Ontario Ministry of Natural Resources, Kemptville, Ontario. iii + 107 pp.
Kerr, S. J. 2000a. Brook trout stocking: An annotated bibliography and literature review with an emphasis on Ontario waters. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 166 pp., appendices (www.mnr.gov.on.ca/MNR/pubs/brook_bio.pdf).
Kerr, S. J. 2000b. F1 splake: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 79 pp., appendices (www.mnr.gov.on.ca/MNR/pubs/splake_bio.pdf).
Kerr, S. J. 2001a. Atlas of muskellunge streams and rivers in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 18 pp.
Kerr, S. J. 2001b. Records of muskellunge (Esox masquinongy) stocking in Ontario, 1900-1999. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Kemptville, Ontario.
Kerr, S. J. 2002a. Atlas of brown trout waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 10 pp. (www.mnr.gov.on.ca/MNR/pubs/BrownTrout_bibliog3.pdf).
Kerr, S. J. 2002b. Atlas of sauger waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 11 pp.
Kerr, S. J. 2002c. Atlas of lake sturgeon waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 12 pp.
Kerr, S. J. 2002d. Fish stocking activities under the Community Fisheries Involvement Program (CFIP), 1982-2001. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. Unpaginated.
Kerr, S. J. 2003a. Atlas of channel catfish waters in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 8 pp.
Kerr, S. J. 2003b. Atlas of brook trout streams and rivers in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. viii + 93 pp.
Kerr, S. J. 2007. Characteristics of Ontario muskellunge (Esox masquinongy) fisheries based on volunteer angler diary information. Environmental Biology of Fishes, 79(1-2):61-69.
Kerr, S. J. 2004. Characteristics of Ontario muskellunge fisheries based on volunteer angler diary information. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 19 pp., appendices I-II.
Kerr, S. J. 2010a. Fish and fisheries management in Ontario: A chronology of events. Biodiversity Branch, Ontario Ministry of Natural Resources, Peterborough. vii + 80 pp., 19 appendices.
Kerr, S. J. 2010b. Muskellunge of the Ottawa River. Fisheries Policy Section, Biodiversity Branch, Ontario Ministry of Natural Resources, Peterborough. ii + 21 pp., 7 appendices.
Kerr, S. J. 2011. Distribution and management of muskellunge in North America: An overview. Fisheries Policy Section, Biodiversity Branch, Ontario Ministry of Natural Resources, Peterborough. ii + 22 pp., 1 appendix.
Kerr, S. J. and R. Cholmondeley. 1992. Results of an aerial creel survey on the Rideau and Cataraqui River systems, summer 1990. Ontario Ministry of Natural Resources, unpublished report. 16 pp.
Kerr, S. J. and R. F. Cholmondeley. 1998. A survey of angling activity on a set of inland lakes in southeastern Ontario during the winter of 1997-98. File Report, Southcentral Sciences Section, Ontario Ministry of Natural Resources, Kemptville, Ontario. 16 pp. + appendices.
Kerr, S. J., M. J. Davison and E. Funnell. 2011. A review of lake sturgeon habitat requirements and strategies to protect and enhance sturgeon habitat. Fisheries Policy Section, Biodiversity Branch, Ontario Ministry of Natural Resources, Peterborough. iv + 58 pp., 4 appendices.
Kerr, S. J. and P. E. Ihssen and B. Sloan. 1998. An annotated bibliography of selected walleye stocking, genetic and stocking assessment references. Walleye Stocking Working Group, Percid Community Synthesis, Ontario Ministry of Natural Resources, Peterborough, Ontario. 194 pp.
Kerr, S. J. and T. A. Lasenby. 2000. Rainbow trout stocking in inland lakes and streams: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. i + 220 pp., appendices.
Kerr, S. J. and T. A. Lasenby. 2001a. Esocid stocking: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 138 pp., 3 appendices.
Kerr, S. J. and T. A. Lasenby. 2001b. Lake trout stocking in inland lakes: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 178 pp., appendices (www.mnr.gov.on.ca/MNR/pubs/LakeTrout_bibliog.pdf).
L
Lacoursière, A., D. J. Graham and R. Poulin.1989. Analyse écologique de huit cours d'eau du parc de la Gatineau. Report by Dryade, Les Saules, Québec for Commission de la Capitale Nationale/National Capital Commission, Ottawa. iv + 55 pp., 3 annexes.
Lafrance, J. M. 1974. Etude de la reproduction du brochet Esox lucius dans la frayère Pélissier, lac McGregor, comté Papineau, 1974. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. iv + 67 pp.
Lafrance, J. M. 1975. Etude de la reproduction du brochet Esox lucius dans la frayère Pélissier, lac McGregor, comté Papineau. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 32 pp. (N)
Lafrance, J. M. 1976. Etude de la reproduction du brochet Esox lucius dans la frayère Pélissier, lac McGregor, comté Papineau. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 33 pp. (N)
Lafrance, J. M. et C. M. Veilleux. 1970. Etude de la reproduction du brochet Esox lucius dans la frayère Pélissier, lac McGregor, comté Papineau.Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 27 pp. (N)
Lafrance, J. M., C. M. Veilleux et P. Gagnon. 1971. Etude de la reproduction du brochet Esox lucius dans la frayère Pélissier, lac McGregor, comté Papineau. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 58 pp. (N)
Lajoie, L. 1986. Statut taxonomique de l'éperlan "nain" dulçaquicole (Pisces; Osmerus) au Québec. Thèse de maîtrise, Université d'Ottawa, Ottawa, Ontario. xi + 137 pp., annexe.
Lalancette, M. 1990. Physico-chimie et inventaire des espèces de poissons fréquentant à l'été 1988 deux marais aménagés par Canards Illimitées et deux baies non aménagées de la rivière des Outaouais. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. v + 39 pp.
Lalancette, M. et C. Sélic. 1988. Inventaire des espèces de poissons fréquentant deux marais aménagés par Canards Illimités et deux baies non aménagées en été sur la rivière des Outaouais en 1988. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Tourisme, de la Chasse et de la Pêche du Québec, Hull. 82 pp., annexes.
Lalancette, M. et G. Thellen. 1992. Comparaison de la mortalité de poissons au cours de l'hiver 1987-1988 dans les baies aménagées et non aménagées pour la faune en bordure de la rive Nord de la rivière des Outaouais entre Hull et Montéllo. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 22 pp., annexes.
Lanteigne, J. 1981. The taxonomy and distribution of the North American lamprey genus Ichthyomyzon. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. xi + 150 pp.
Lanteigne, J. 1992. Status of the northern brook lamprey, Ichthyomyzon fossor, in Canada. Canadian Field-Naturalist, 106(1):7-13.
Lanteigne, J., J. M. Hanson and S. U. Qadri. 1981. Occurrence and growth patterns of the American brook lamprey, Lethenteron lamottenii, in the Ottawa River. Canadian Field-Naturalist, 95(3):261-265.
Lanteigne, J. and D. E. McAllister. 1983. The pygmy smelt, Osmerus spectrum Cope, 1870, a forgotten sibling species of eastern North American fish. Syllogeus, Ottawa, 45:1-32.
Lapierre, K. and B. W. Coad. 2006. Review of “Fishes of the Great Lakes Region Revised Edition” by Carl L. Hubbs and Karl F. Lagler. Revised by Gerald R. Smith. 2004. The University of Michigan Press, Ann Arbor, Michigan, USA. xvii + 276 pages, plates 1-32. $24.95. Canadian Field-Naturalist, 119(2)(2005):301-302 (http://www.ofnc.ca/cfn/119-2/21_BookReviews119(2).pdf).
Lapointe, M. 1997. Rapport sur la situation du fouille-roche gris (Percina copelandi) au Québec. Direction de la faune et des habitats, Ministère de l’Environnement et de la Faune, Québec. 55 pp.
Lasenby, T. A. and S. J. Kerr. 2000. Bass stocking and transfers: annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 233 pp. (www.mnr.gov.on.ca/MNR/pubs/Bass_bibliog.pdf).
Lasenby, T. A. and S. J. Kerr. 2001. Brown trout stocking: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 187 pp, 3 appendices.
Lasenby, T. A., S. J. Kerr and G. W. Hooper. 2001. Lake whitefish culture and stocking: An annotated bibliography and literature review. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. ii + 187 pp, 3 appendices.
Laucius, J. 2009. 4-foot-long lunkers lurk in our canal. Ottawa Citizen, 3 March, 2009,
Lauriol, B., E. Deschamps, L. Carrier, W. Grimm, R. Morlan and B. Talon. 2003. Cave infill and associated biotic remains as indicators of Holocene environment in Gatineau Park (Quebec, Canada). Canadian Journal of Earth Sciences, 40(6):789-803.
Lauzon, C. 2003. South Nation River Fisheries Assessment Report 2001-2003. South Nation Conservation de la Nation Sud and Ontario Ministry of Natural Resources. xii + 64 pp.
Lee, D. S., C. R. Gilbert, C. H. Hocutt, R. E. Jenkins, D. E. McAllister and J. R. Stauffer. 1980. Atlas of North American Freshwater Fishes. North Carolina State Museum of Natural History, Raleigh. x + 854 pp.
Legault, R.-O. and C. Delisle. 1967. Acute infection by Glugea hertwigi Weissenberg in young-of-the-year rainbow smelt, Osmerus eperlanus mordax (Mitchill). Canadian Journal of Zoology, 45(6, part 2):1291-1292, plate.
Legault, R.-O. et C. Delisle. 1968. La fraye d'une population d'éperlans géants, Osmerus eperlanus mordax, au lac Heney, Comté de Gatineau, Québec. Journal of the Fisheries Research Board of Canada, 25(9):1813-1830.
Legendre, P. and V. Legendre. 1984. Postglacial dispersal of freshwater fishes in the Québec peninsula. Canadian Journal of Fisheries and Aquatic Sciences, 41(12):1781-1802.
Legendre, V. 1954a. The Freshwater Fishes. Volume 1. Key to game and commercial fishes of the Province of Quebec. First English Edition. Game and Fisheries Department, Quebec. 180 pp.
Legendre, V. 1954b. Les poissons d'eau douce. Tome 1. Clef des poissons de pêche sportive et commerciale de la Province de Québec. Ministère de la Chasse et des Pêcheries, Québec. 180 pp.
Legendre, V. 1960. Les poissons d'eau douce. Tome 2. Clef des Cyprinidés ou Ménés du Québec. Le Jeune Naturaliste, 10(9 et 10):178-212.
Legendre, V. 1971. L'ichthyogéographie du Québec et la documentation corrélative. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Bulletin, 12:1-71.
Leggett, R. 1975. Ottawa Waterway. Gateway to a Continent. University of Toronto Press, Toronto. xi + 291 p., map.
Leggett, R. 1986. Rideau Waterway. 2nd Edition. University of Toronto Press, Toronto. 312 pp., map.
Lemieux, P. 1986. Proposed methodology for the ecological analysis of the major rivers and streams of Gatineau Park. Manuscript Report, Resource Conservation Section, National Capital Commission, Ottawa. iii + 50 pp.
Lepage, S. 1992. Évaluation de la faune ichtyenne dans le marais aux Massettes, au printemps 1992. Entente cadre concernant un plan quinquennial pour la protection et l'aménagement des habitats fauniques. Volet 1.G. Ministère du Tourisme, de la Chasse et de la Pêche du Québec et Canards Illimités. 22 pp. (N)
Lepage, S. et R. Lalumière. 2003. Modalités de gestion du poisson dans les aménagements fauniques en plaine inondable. Société de la faune et des parcs du Québec, Direction du développement de la faune et Groupe conseil GENIVAR inc, Québec. vii + 55 pp.
Léveillé, M. 1988. Plan de pêche de la rivière des Outaouais. Résultats de la campagne de marquage d'esturgeons jaune (Acipenser fulvescens) de la rivière des Outaouais à l'automne 1988. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 10 pp., annexes.
Léveillé, M. 1989. Plan de pêche de la rivière des Outaouais. Détermination d'âge d'esturgeons jaune (Acipenser fulvescens) capturés à la pêche commerciale. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 25 pp., annexes.
Levere, R. 1969. 1969 creel census report Ottawa River. Ontario Department of Natural Resources, Kemptville, Ontario, Manuscript Report. 35 pp.
Levesque, F. et J. Therrien. 1992. Techniques d'échantillonnage pour la surveillance des populations de poissons dans la système du Saint-Laurent: Prétest dans la rivière des Outaouias en 1990. Groupe Environnement Shooner Inc., Rapport présenté au Direction de la gestion des espèces et des habitats et Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Québec. 40 pp., annexes.
Levin, M. 2006. Life interrupted. The Ottawa Citizen, 30 April 2006, p. A8.
Lindeman, D. H. 1987. Rideau River index fishing survey. Ontario Ministry of Natural Resources, unpublished report.
M
MacDonald, E. 1938. Shad still run the Ottawa. Rod and Gun in Canada, 39(9):18, 34-35.
MacKay, H. H. 1963. Fishes of Ontario. Fish and Wildlife Branch, Department of Lands and Forests, Ontario. xi + 300 pp.
MacLean, N. G. 1994. The 1993 Volunteer Muskellunge Angler Diary Program in southeastern Ontario. Southern Region Science and Technology Transfer Unit Information Report IR-004, Ontario Ministry of Natural Resources, Brockville. 13 pp., appendices. (N)
MacLeod, J. and J. Wiltshire. 2004. Atlas of walleye streams and rivers in Ontario. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 44 pp.
MacQuart, M., P. Houde et C. Grondin. 1990. Suivi de certains paramètres physico-chimiques et expérimentation de techniques de pêches dans le marais aux Massettes, rivière des Outaouais, au cours de l'hiver 1989. Entente cadre concernant un plan quinquennal pour la protection et l'aménagement des habitats fauniques. Volet 1.G. Ministère du Loisir, de la Chasse et de la Pêche et Canards Illimités Canada, Québec. 23 pp.
Madill, J. 1988. New Canadian records of leeches (Annelida: Hirudinea) parasitic on fish. Canadian Field-Naturalist, 102(4):685-688.
Magnin, É. and C. Fradette. 1974. Zonage écologique de la région du Parc de la Gatineau. Préparé pour la Commission de la Capitale nationale. Rapport final. Cahier no. 2: Physico-chimie, flore et faune des eaux de la Gatineau (examen des données existantes et conclusion). Interpretation Service/Service d'Interprétation, National Capital Commission/Commission de la Capitale nationale, Chelsea.
Mandrak, N. E. 1990. The zoogeography of Ontario freshwater fishes. M.Sc. Thesis, University of Toronto, Toronto, Ontario. (O)
Mandrak, N. E. 1995. Biogeographic patterns of fish species richness in Ontario lakes in relation to historical and environmental factors. Canadian Journal of Fisheries and Aquatic Sciences, 52(7):1462-1474.
Mandrak, N. E. and E. J. Crossman. 1992a. A checklist of Ontario freshwater fishes annotated with distribution maps. Royal Ontario Museum, Toronto. v + 176 pp.
Mandrak, N. E. and E. J. Crossman. 1992b. Postglacial dispersal of freshwater fishes into Ontario. Canadian Journal of Zoology, 70(11):2247-2259.
Mandrak, N. E. and W. H. Ramshaw. 1998. Status of the eastern silvery minnow, Hybognathus regius, in Canada. Canadian Field-Naturalist, 112(1):141-146.
Marleau, J., R. Mussio and W. Mussio. 2002. Fishing Ontario. Eastern Ontario. Mussio Ventures Ltd., New Westminster, B.C. 104 pp.
McAllister, D. E. 1968. A list of the fishes of the Ottawa area. Trail & Landscape, 2(6):148-153.
McAllister, D. E. 1971. Iowa darters (Etheostoma exile) spawning at Lac Lapeche, Quebec. Trail & Landscape, 5(4):112-113.
McAllister, D. E. 1990. A List of the Fishes of Canada/Liste des poissons du Canada. Syllogeus, Ottawa, 64:1-310.
McAllister, D. E. and B. W. Coad. 1975a. Fishes of Canada's National Capital Region/Poissons de la région de la capital du Canada. Fisheries Research Board of Canada Miscellaneous Special Publication, 24:1-200 (dated 1974).
McAllister, D. E. and B. W. Coad. 1975b. Fishes of Canada's National Capital Region [excerpts from the Introduction]. Trail & Landscape, 9(4):117-121. (Excerpted from McAllister and Coad, 1975a).
McAllister, D. E. and B. W. Coad. 1975c. Mooneye Hiodon tergisus Le Sueur. Trail & Landscape, 9(5):146-147. (Excerpted from McAllister and Coad 1975a).
McAllister, D. E. and B. W. Coad. 1976a. Lake Charr (trout) Salvelinus namayoush (sic) (Walbaum). Trail & Landscape, 10(1):20-21. (Excerpted from McAllister and Coad, 1975a).
McAllister, D. E. and B. W. Coad. 1976b. Longnose dace Rhinichthys cataractae (Valenciennes). Trail & Landscape, 10(2):42-43. (Excerpted from McAllister and Coad, 1975a).
McAllister, D. E. and B. W. Coad. 1976c. Tadpole madtom Noturus gyrinus (Mitchill). Trail & Landscape, 10(3):56-57. (Excerpted from McAllister and Coad 1975a).
McAllister, D. E. and B. W. Coad. 1976d. Brook stickleback Culaea inconstans (Kirtland). Trail & Landscape, 10(4):90-91. (Excerpted from McAllister and Coad, 1975a).
McAllister, D. E. and B. W. Coad. 1976e. Common shiner Notropis cornutus (Mitchill). Trail & Landscape, 10(5):118-119. (Excerpted from McAllister and Coad, 1975a).
McAllister, D. E., B. W. Coad, P. Rubec and J. Aniskowicz. 1974. Fishes of Canada's National Capital Region / Poisson de la région de la capital du Canada. Program of the 54th Annual Meeting, American Society of Ichthyologists and Herpetologists, National Museum of Natural Sciences, Ottawa, 17-22 June 1974, p. 12-13.
McAllister, D. E. and E. J. Crossman. 1973. A Guide to the Freshwater Sport Fishes of Canada. National Museum of Natural Sciences, Natural History Series, 1:xi + 89 pp.
McAllister, D. E., S. L. Cumbaa and C. R. Harington. 1981. Pleistocene fishes (Coregonus, Osmerus, Microgadus, Gasterosteus) from Green Creek, Ontario, Canada. Canadian Journal of Earth Sciences, 18(8):1356-1364.
McAllister, Don E. and C. G. Gruchy. 1977. Status and habitat of Canadian fishes in 1976, p. 151-157. In: Mosquin, T. and Suchal, C. (Eds.). Proceedings of the Symposium on Canada's Threatened Species and Habitats. Canadian Nature Federation, Special Publication, Ottawa, 6:1-185.
McAllister, D. E., C. R. Harington, S. L. Cumbaa and C. B. Renaud. 1987. Paleoenvironmental and biogeographic analyses of fossil fishes in peri-Champlain Sea deposits in eastern Canada. In: Gadd, N. R. (Ed.). The Late Quaternary Development of the Champlain Sea Basin. Special Papers of the Geological Association of Canada, 35:241-258.
McAllister, D. E., P. Jolicoeur and H. Tsuyuki. 1972. Morphological and myogen comparison of johnny and tessellated darters and their hybrids, genus Etheostoma, near Ottawa, Canada. Journal of the Fisheries Research Board of Canada, 29(8):1173-1180.
McAllister, D. E., B. J. Parker and P. M. McKee. 1985. Rare, endangered and extinct fishes in Canada. Syllogeus, Ottawa, 54:1-192.
McCrimmon, H. R. 1968. Carp in Canada. Bulletin of the Fisheries Research Board of Canada, 165:ix + 93 pp.
McCuaig, J. 1999. 'Fishing not recommended'. The Ottawa Citizen, 24 October 1999, p. A17 (reprint of letter from 19 June 1935).
McKinnon, A. E., R. J. Norstrom and A. S. W. deFreitas. 1975. Bioaccumulation of methyl mercury: a predictive model with parameters for Ottawa River yellow perch Perca flavescens. International Conference on Heavy Metals in the Environment, Toronto, Ontario, 27-31 October 1975 (title).
McMaster, M. E., K. R. Munkittrick, C. Cobden and M. Groves. 2001. Field studies examining reproductive function in feral fish downstream of the Bowater Pulp and Paper Canada Inc. newsprint mill in Gatineau, Quebec. Environment Canada, National Water Research Institute, Burlington/Saskatoon, AEP-TN01-058.
McMaster, M. E., K. R. Munkittrick and D. Lemire. 2001. Papier Masson Mill field studies examining reproductive function in feral fish - research program for cycle II of the environmental effects monitoring program for the Masson Mill in Masson, Quebec. Environment Canada, National Water Research Institute, Burlington/Saskatoon, AEP-TN01-056.
McMaster, M. E., K. R. Munkittrick, C. Wood and R. Riffon. 2001. Maclaren Mill field studies examining reproductive function in feral fish - research program for cycle II of the environmental effects monitoring program for the Thurso Mill in Thurso, Quebec. Environment Canada, National Water Research Institute, Burlington/Saskatoon, AEP-TN01-057.
McMurray, I. T. 1984. A Herpetofaunal Study of Gatineau Park. Report to the National Capital Commission, Ottawa. viii + 943 pp. (5 volumes).
McPhail, J. D. 1963. Geographic variation in North American ninespine sticklebacks, Pungitius pungitius. Journal of the Fisheries Research Board of Canada, 20:27-44.
Meeking, D. 1989. The Rideau muskellunge spawning survey. Internal Report, Ottawa Valley Chapter, Muskies Canada.
Mélançon, C. 1973. Les poissons de nos eaux. Quatrième Édition, revue et augmentée. Éditions du Jour, Montréal. 455 pp.
Metcalfe, J. C. 1970. Minnows by the millions. Ottawa Journal, 27 June 1970.
Migneault, J.G. 1981. Résultats de la pêche expérimentale dans la rivière des Outaouais entre Bristol et l'estuaire de la rivière Dumoine, en 1980. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 17 pp., annexe 1
Migneault, J. G. 1985. Evaluation de la rentabilité de la frayère à grand brochet dans le lac McGregor, comté Papineau, rapport d'étape. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec. 32 pp. (N)
Millette, F. J. 1991. Rideau Canal Conservation Plan. Rideau canal conservation Plan. Canadian Parks Service, Rideau Canal Resource Conservation Section, Smiths Falls, Ontario. ix + 71 pp.
Moisan, M. 1998. Rapport sur la situation du chevalier de rivière (Moxostoma carinatum) au Québec. Direction de la faune et des habitats, Ministère de l’Environnement et de la Faune, Québec. xiii + 73 pp.
Moisan, M. and H. Laflamme. 1999. Rapport sur la situation de l'esturgeon jaune (Acipenser fulvescens) au Québec. Direction de la faune et des habitats, Faune et Parcs Québec, Québec. xi + 68 pp.
Mongeau, J.-R., A. Courtemanche, G. Massé et B. Vincent. 1974. Cartes de répartition géographique des espèces de poissons du sud du Québec, d'après les inventaires ichthyologiques effectués de 1963 à 1972. Faune du Québec, Rapport Spécial, Service de l'Aménagement de la Faune, Direction de la Chasse et de la Pêche, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, 4:xvii + 92 pp.
Morrison, J. 2004. Biologists fishing for sturgeon info. Ottawa Sun Online, 27 May 2004, 3 pp.
Mullington, D. 1998. Muskie catch one of largest of year in North America: Rockland renowned as one of the best places on Ottawa for big fish. The Ottawa Citizen, 13 October 1998, p. C1.
Mullington, D. 1999. Giant muskie elderly, but feisty. The Ottawa Citizen, 10 December 1999, C9.
N
Nash, C. W. 1908. Check list of the vertebrates of Ontario and catalogue of specimens in the Biological Section of the Provincial Museum. Department of Education, Toronto. 122 pp.
Neave, F. B. 2004. The utility of meristic, morphometric, pigmentation and gonad analysis in the identification of Ichthyomyzon lamprey larvae. M.Sc. Thesis, Faculty of Graduate Studies, University of Guelph, Ontario. iii + 120 pp.
Neave, F. B., N. E. Mandrak, M. F. Docker and D. L. Noakes. 2007. An attempt to differentiate sympatric Ichthyomyzon ammocoetes using meristic, morphological, pigmentation, and gonad analyses. Canadian Journal of Zoology, 85(4):549-560.
Nelson J. S. 1994. Fishes of the World. 3rd Edition. John Wiley & Sons, New York. xvii + 600 pp.
Nelson, J. S. 2006. Fishes of the World. Fourth Edition. John Wiley & Sons, New York. xix + 601 pp.
Nelson, J. S., E. J. Crossman, H. Espinosa-Pérez, L. T. Findley, C. R. Gilbert, R. N. Lea and J. D. Williams. 2004. Common and Scientific Names of Fishes from the United States, Canada, and Mexico. American Fisheries Society Special Publication, 29:ix + 386 pp.
Niblett Environmental Associates Inc. 1990. Chats Falls GS Sluice Gate Study. Consultants report to W. Weller, Ontario Hydro Safety and Environment Department (file no. EAA 12.31.1). 11 pp.
Niblett Environmental Associates Inc. 1991. City of Gloucester River Ridge Environmental Summary. A Report for the Development Department, Corporation of the City of Gloucester by Niblett Environmental Associates Inc. iv + 26 pp.
Niblett Environmental Associates Inc. 1992. South Urban Community City of Gloucester - technical data report (cited in below).
Niblett Environmental Associates Inc. 1992a. South Urban Community City of Gloucester stormwater management environmental evaluation. A Report for the Development Department, Corporation of the City of Gloucester by Niblett Environmental Associates Inc. i + 55 pp.
Niblett Environmental Associates Inc. 1992b. Addendum Report. Rideau River and Mosquito Creek: fish mortality and habitat alterations observations. A Report for the Corporation of the City of Gloucester by Niblett Environmental Associates Inc. 15 pp.
Nicol, I. 1981. Brook stickleback, a fish that builds a nest. Trail & Landscape, 15(4):216-217.
Norstrom, R. J., A. E. McKinnon and A. S. W. DeFreitas. 1976. A bioenergetics-based model for pollutant accumulation by fish. Simulation of PCB and methylmercury residue levels in Ottawa River yellow perch (Perca flavescens). Journal of the Fisheries Research Board of Canada, 33(2):248-267.
Norstrom, R. J., M. Brownstein and A. S. W. deFrietas. 1974. Chemical analysis, 1972-1973 and fish bioaccumulation model. Report 19A:1-50. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 2, Ottawa River Project. University of Ottawa-Division of Biological Sciences, National Research Council of Canada, Ottawa, Ontario. v + 602 pp.
O
Ontario Ministry of the Environment. 1977. Ontario Ministry of the Environment technical report on the Mississippi River, Lanark, Ontario. Ontario Ministry of the Environment, Toronto.
Ontario Ministry of Natural Resources (OMNR). 1988. Carleton Place District fisheries mangement plan, 1987-2000. Carleton Place District, Ontario Ministry of Natural Resources, Carleton Place, Ontario. 118 pp.
Ontario Ministry of Natural Resources (OMNR). 2000. Rideau River Fisheries Assessment Report 1998-1999: Index Netting & Contaminant Analysis, Kilmarnock & Andrewsville Reaches. Ontario Ministry of Natural Resources, Kemptville, Ontario. 34 pp. (N)
Ontario Ministry of Natural Resources (OMNR). 2002. Proposal for managing the sport fishery for muskellunge in Ontario. Draft. Ontario Ministry of Natural Resources, Peterborough, Ontario. 8 pp.
Ontario Ministry of Natural Resources (OMNR) and Gouvernement du Québec Faune et Parcs. 1999. A strategic fisheries management framework for the Ottawa River. Ontario Ministry of Natural Resources, Pembroke, Ontario. 72 pp.
Ontario Ministry of Natural Resources (OMNR) and Rideau Valley Conservation Authority (RVCA). 1976. Fishing on Ottawa's doorstep. OMNR and RVCA. 26 pp.
Osterberg, D. M. 1978. Food consumption, feeding habits, and growth of walleye (Stizostedion vitreum) and sauger (Stizostedion canadense) in the Ottawa River near Ottawa-Hull, Canada. Ph.D. Thesis, University of Ottawa, Ottawa, Ontario xiii + 136 pp.
O'Toole, A., R. Walker, Z. Whynot, A. Gingerich, K. Hanson, and S.J. Cooke. 2006. Fisheries and fish habitat assessment for the Rideau Canal, Dow's Lake location of work with respect to the open cut tunnel for the North-South Light Rail Project Interim Report: Objectives, methods, progress, and future directions. Fish Ecology and Conservation Physiology Laboratory Research Report Series, Carleton University, Ottawa, 06-02:1-30.
Ottawa River Project. 1974. Distribution and transport of pollutants in flowing water ecosystems. Report Number 2, Ottawa River Project. University of Ottawa-Division of Biological Sciences, National Research Council of Canada, Ottawa, Ontario. v + 602 pp.
Ottawa River Project. 1976. Distribution and transport of pollutants in flowing water ecosystems. University of Ottawa - National Research Council of Canada, Report No. 3. 1 volume.
Ottawa River Project. 1977. Distribution and transport of pollutants in flowing water ecosystems. University of Ottawa - National Research Council of Canada, Final Report, 2 volumes.
Ottawa River Project Group. 1977. Mercury levels in fish from the Ottawa River. Chapter 31:1-30. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Final Report Volume 2, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Ottawa River Project Group. 1979. Mercury in the Ottawa River. Environmental Research, 19(2):231-243.
Ottawa Riverkeeper/Sentinelle Outaouais. 2006. Ottawa Riverkeeper's River Report: Issue No.1, Ecology and Impacts. Ottawa. 83 pp.
Otto, D. M. E. and T. W. Moon. 1996. Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and non-polluted systems. Archives of Environmental Contamination and Toxicology, 31(1):141-147.
P
Page, L. M. and B. M. Burr. 1991. A Field Guide to Freshwater Fishes of North America North of Mexico. Houghton Mifflin Company, Boston (Peterson Field Guide Series 42). xii + 432 pp.
Paradis, A. R. et F. Chapleau. 1994. Impact de la maladie des points noirs sur la biologie du complexe Phoxinus (Cyprinidae) du lac Fortune, Québec. Canadian Journal of Zoology, 72(9):1611-1615.
Pariseau, R. et P. Dumont. 1980. Inventaire ichthyologique de la rivière des Outaouais (1979). Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 17 pp.
Pariseau, R., P. Dumont et J.-G. Migneault. 1983. Découverte, dans le sud-ouest du Québec, d'une population de cisco de lac, Coregonus artedii, frayant au printemps. Canadian Journal of Zoology, 61(10):2365-2368.
Pariseau, R. et H. Fournier. Poissons capturés au rapide Farmers, premier rapide de la rivière Gatineau en amont de sa confluence avec la rivière des Outaouais, au printemps 1999. En preparation (sic, not located).
Pariseau, R., H. Fournier et P. Dumont. 1982. Enquête auprès des vendeurs de poissons-appâts de la région administrative de l'Outaouais. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 17 pp. (N)
Pariseau, R., H. Fournier, J-P. Harnois and G. Michon. 2007. Recherche de fouille-roche gris (Percina copelandi), et de méné d'herbe (Notropis bifrenatus) dans la rivière des Outaouais entre Carillon et Rapides-des-Joachims. Ministère des Ressources naturelles et de la Fauna, Région de l'Outaouais, Gatineau. iii + 20 pp.
Parker, B. J. 1988. Updated status of the river redhorse, Moxostoma carinatum, in Canada. Canadian Field-Naturalist, 102(1):140-146.
Parker, B. and P. McKee. 1980. Rare, threatened and endangered fishes in southern Ontario: Status reports. Report for the Department of Supply and Services, Department of Fisheries and Oceans and the National Museum of Natural Sciences, Ottawa by Beak Consultants, Mississauga.
Parker, B. and P. McKee. 1984. Status of the river redhorse, Moxostoma carinatum, in Canada. Canadian Field-Naturalist, 98(1):110-114.
Parks Canada. 1996. Rideau Canal Management Plan. Canadian Heritage, Parks Canada. v + 82 pp.
Paul, M. et D. Laliberté. 1986. Réseau de surveillance des substances toxiaques 1983: contamination par le mercure des poissons et des sédiments de six bassins versants du Québec méridional. Direction des relevés aquatiques, Ministère de l'Environnement, Québec, Rapport 85-10:1-68.
Paul, M. et D. Laliberté. 1987. Teneur en BCP, pesticides, organochlorés, composés phénoliques et HAP des poissons et des sédiments du bassin versant de la rivière des Outaouais en 1985. Direction de la qualité du milieu aquatique, Ministère de l'Environnement, Québec, Rapport QE86-10:1-85.
Penney, L. 1991. A summary of Muskies Canada Inc. (Ottawa Valley Chapter) logs and catch records (1988-1990) for the Rideau, Ottawa, and St. Lawrence rivers. Ontario Ministry of Natural Resources, Kemptville, Ontario. 20 pp.
Pépin, S., C. Dubé et C. Grondin. 1991. Utilisation d'un marais aménagé pour la sauvagine et d'un marais naturel par la faune ichtyenne de la rivière des Outaouais. Entente cadre concernant un plan quinquennal pour la protection et l'aménagement des habitats fauniques. Volet 1.G. Ministère du Loisir, de la Chasse et de la Pêche et Canards Illimités Canada, Québec. 156 pp.
Pépin, S., A. Tremblay et C. Grondin. 1990. Évaluation de techniques de pêche expérimentale en milieux humides, inventaire ichtyologique et caractérisation des habitats dans le marais aux Massettes (région de l'Outaouais). Entente cadre concernant un plan quinquennal pour la protection et l'aménagement des habitats fauniques. Volet 1.G. Ministère du Loisir, de la Chasse et de la Pêche et Canards Illimités Canada, Québec. 82 pp.
Peterson, D., P. Vecsei and D.L.G. Noakes. 2003. Threatened fishes of the world: Acipenser fulvescens Rafinesque, 1817 (Acipenseridae). Environmental Biology of Fishes, 68(2):174.
Phelps A. M. 1999. Investigating the relationship between the Rideau River (Ontario, Canada) fish community and the habitat characteristics associated with land use. 79th Annual Meeting of the American Society of Ichthyologists and Herpetologists, Pennsylvania State University, Philadelphia, U.S.A., June 24-30, Program Book and Abstracts, p. 184 (abstract).
Phelps, A-M. 2001. Investigating the fish community of the Rideau River, Ontario, with respect to historical changes and current land-use practices. M.Sc. Thesis, Department of Biology, University of Ottawa, Ottawa, Ontario. xv + 176 pp.
Phelps, A. and A. Francis. 2000. Updated COSEWIC Status Report on margined madtom Noturus insignis. Report submitted to the Committee on the Status of Endangered Wildlife in Canada. 20 pp.
Phelps, A., C. B. Renaud and F. Chapleau. 1999a. Over one hundred years of change within the fish community of the Rideau River/Canal system, Ontario, Canada. 38th Annual Meeting of the Canadian Society of Zoologists, University of Ottawa, Ottawa, May 5-8 (abstract).
Phelps, A., C. B. Renaud and F. Chapleau. 1999b. Étude comparative des communautés de poissons de la rivière Rideau en milieu agricole, urbain et forestier. Colloque sur La biodiversité de la rivière Rideau: la science à la portée du public, 67è Congrès de l’Association canadienne-française pour l’avancement des sciences, University of Ottawa, Ottawa, May 10-14. (abstract).
Phelps, A., C. B. Renaud and F. Chapleau. 2000. First record of a freshwater drum, Aplodinotus grunniens, in the Rideau River, Ottawa, Ontario. Canadian Field-Naturalist, 114(1):121-125.
Phelps, A., F. Chapleau and C. B. Renaud. 2000. The tadpole madtom, Noturus gyrinus. A rarely seen fish of the Rideau River system, Ontario. Trail & Landscape, 34(1):30-34.
Phelps, A. and A. Francis. 2002. Update COSEWIC status report on the margined madtom Noturus insignis in Canada, in COSEWIC assessment and update status report on the margined madtom Noturus insignis in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. vii + 17 pp.
Pluritec Ltée. 1982a. Inventaire ecologique de lacs - 1982. Parc de la Gatineau. Report by Pluritec Ltée for the Gatineau Park, National Capital Commission, Ottawa. 47 pp., appendices 1-7, maps.
Pluritec Ltée. 1982b. Historique de la peche commerciale en eaux douces depuis 1950. Report by Pluritec Ltée for the Ministère du Loisir, de la Chasse et de la Pêche, Québec. 250 pp.
Poulin, M. 2001. A multidisciplinary, community-based study of the environmental health of the Rideau River: final report. Report presented to the EJLB Foundation, Montreal. 46 pp.
Preece, J. G. 2001. State of the River Report. A Report on environmental health of the Rideau River. Report for the Research and Monitoring Committee of the Rideau River Roundtable, Ottawa. vii + 81 pp. (www.rideauvalley.on.ca/programs/rrr/rrr.html, downloaded 8 May 2003).
Prince, E. E. and A. Halkett. 1906. The eggs of the fresh-water ling. The Ottawa Naturalist, 19(12):219-224.
Prince, E. E., A. Halkett, W. S. Odell and E. E. Lemieux. 1906. Zoological Report - 1905-6. The Ottawa Naturalist, 20(2):56-61.
Prince, E. E., A. Halkett, W. S. Odell and E. E. Lemieux. 1908. Report of the Zoological Branch, 1907. The Ottawa Naturalist, 21(10):198-201.
Provost, J., L. Verret et P. Dumont. 1984. L'alose savoureuse au Québec: synthèse des connaissances biologiques et perspectives d'aménagement d'habitats. Rapport manuscrit canadien des sciences halieutiques et aquatiques, 1793:xii + 114 pp.
Punt, K. and V. Castro. 2006. Ottawa River fish-kill event August of 2006. Information Report, Ontario Ministry of Natural Resources, Pembroke and Kingston. 18 pp., 5 appendices.
Qadri, S. U. and B. W. Coad. 1974. Abundance and distribution of small sized fish in the Ottawa River. Report 17:1-15. In: Ottawa River Project. Distribution and transport of pollutants in flowing water
Qadri, S. U. and D. W. Rodgers. 1976. Food habits, growth, energy transformations and pollutant bioaccumulation of yearling yellow perch (Perca flavescens). Chapter 12:1-25. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 3, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Qadri, S. U. and D. W. Rodgers. 1977. Food habits, growth, energy transformations and pollutant accumulation of yearling yellow perch (Perca flavescens). Chapter 29:1-24. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Final Report Volume 2, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Qadri, S. U. and P. J. Rubec. 1974. Age, growth and population density of brown bullhead, northern pike, walleye, sauger and yellow perch in a 3 mile section of the Ottawa River. Report 16:1-60. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Report No. 2, Ottawa River Project. University of Ottawa-Division of Biological Sciences, National Research Council of Canada, Ottawa, Ontario. v + 602 pp.
Qadri, S. U. and P. J. Rubec. 1977. Age, growth and population density of brown bullhead, northern pike, walleye, sauger and yellow perch in a 3 mile section of the Ottawa River. Chapter 28:1-60. In: Ottawa River Project. Distribution and transport of pollutants in flowing water ecosystems. Final Report Volume 2, Ottawa River Project. University of Ottawa-National Research Council of Canada, Ottawa, Ontario.
Québec Ministère de la Santé et des Services Sociaux, Ministère de l'Environnement, et Centre de Toxicologie du Québec. 1992. Guide de consommation du poisson de pêche sportive en eau douce. Gouvernement du Québec. 80 pp.
R
Radforth, I. 1944. Some consideration on the distribution of fishes in Ontario. Royal Ontario Museum Zoology, 25:1-116.
Raine, G. E. 1968. 1967-68 winter fishing report for the Kemptville administrative district. File Report, Ontario Ministry of Natural Resources, Kemptville, Ontario. 17 pp.
Raine, G. E. 1970a. 1969-70 winter fishing report for the Kemptville administrative district. File Report, Ontario Ministry of Natural Resources, Kemptville, Ontario.
Raine, G. E. 1970b. 1970 summer fishing report for the Kemptville administrative district. File Report, Ontario Ministry of Natural Resources, Kemptville, Ontario. 5 pp.
Ready, L. E. 1973. Operation Bayou, Phase 1. Carleton Place High School, Ontario. 37 pp.
Reid, S. M. 2008. Comparison of scales, pectoral fin rays and opercles for age estimation on Ontario redhorse, Moxostoma species. Canadian Field-Naturalist, 121(1)(2007):29-34.
Renaud, C. and J. Berkowitz. 1999. Museum scientist receives an oscar. Press Release, Canadian Museum of Nature/Musée canadian de la Nature, Ottawa, 14 July 1999, 2 pp.
Renaud, C. B. 2003. The muskellunge, Esox masquinongy, as a host for the silver lamprey, Ichthyomyzon unicuspis, in the Ottawa River, Ontario/Québec. Canadian Field-Naturalist, 116(3)(2002):433-440.
Renaud, C. B. and N. de Ville. 2000. Three records of the Chestnut Lamprey, Ichthyomyzon castaneus, new to Québec. Canadian Field-Naturalist, 114(2):333-335.
Renaud, C. B. and A. Phelps. 1999. Oscar-winning catch. Trail & Landscape, 33(4):178-180.
Renaud, C. B. and A. Phelps. 2001. A pacu/piranha in the Rideau Canal. Trail & Landscape, 35(2):86-89.
Réseau d'observation active de la Biosphère. 1996. Rapport de pêche 1995-1996. Pathologie des poissons d'eau douce des écosystèmes Saint-Laurent-Grands Lacs rives de l'Outaouais région d'Ottawa-Carleton. Réseau d'observation active de la Biosphère, Montréal. 18pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Réseau d'observation active de la Biosphère. 1997. Rapport de pêche 1996-1997. Observation de la cécité et du parasitisme des poissons rives de l'Outaouais, région d'Ottawa-Carleton. Réseau d'observation active de la Biosphère, Montréal et Mini-cours d'enrichissement-sciences, Faculté d'éducation, Université d'Ottawa, Ottawa. 8 pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Réseau d'observation active de la Biosphère. 1998. Réseau d'observation des poissons d'eau douce (ROPED) Rivière des Outaouais. Réseau d'observation active de la Biosphère, Montréal et Mini-cours d'enrichissement-sciences, Faculté d'éducation, Université d'Ottawa, Ottawa. 13 pp. (Freshwater Fish Ecowatch Network, Environment Canada (www.biosphere.ec.gc.ca).
Rheault, J-P. 2005. Fish of the month: margined madtom. Les Amis du Parc de la Gatineau/Friends of Gatineau Park, Summer 2005 Newsletter, p. 3.
Richards, J. L. & Associates Limited. 1995. Britannia Mud Lake. A Four year Monitoring Program. Summary Report of the 1994 Baseline Data. Report by J. L. Richards & Associates Limited, Ottawa to the City of Ottawa, Project No. 50057. 37 pp.
Richards, J. L. & Associates Limited. 1997. Britannia storm sewer, outlet and sedimentation facilities, long term monitoring and impact assessment, Mud Lake monitoring program. Report by Richards, J. L. & Associates Limited, Ottawa to the Department of Engineering and Works, City of Ottawa. Project JLR 13980-21. ii + 31 pp.
Ridgway, L. L. and F. Chapleau. 1994. Study of a stunted population of yellow perch (Perca flavescens) in a monospecific lake in Gatineau Park. Canadian Journal of Zoology, 72(9):1576-1582.
RMOC (Regional Municipality of Ottawa-Carleton). 1995a. An investigation of the benthic macroinvertebrates and fish communities of Mooney's Bay. Draft Report. Surface Water Quality Branch, RMOC, Ottawa. 85 pp.
RMOC (Regional Municipality of Ottawa-Carleton). 1995b. Rideau River fisheries assessment. 1995 Summary report. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, RMOC, Ottawa. vi + 65 pp.
RMOC (Regional Municipality of Ottawa-Carleton). 1996a. Rideau River fisheries assessment. 1996 Spring monitoring report. Internal draft. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, RMOC, Ottawa. iv + 27 pp.
RMOC (Regional Municipality of Ottawa-Carleton). 1996b. Rideau River fisheries assessment. 1996 Summer monitoring report. Internal draft. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, RMOC, Ottawa. ii + 34 pp.
RMOC (Regional Municipality of Ottawa-Carleton). 1997. Proposed Rideau River fisheries assessment program 1997. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, RMOC, Ottawa. 9 pp.
RMOC (Regional Municipality of Ottawa-Carleton). 1998a. Rideau River fisheries assessment report (index netting, nursery habitat inventory, contaminant and fall drawdown monitoring) 1995-1997. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, RMOC, Ottawa. viii + 62 pp. (authored by A. Bendig).
RMOC (Regional Municipality of Ottawa-Carleton). 1998b. Regional Profile. Planning and Development Approvals Department #6-12.
RMOC (Regional Municipality of Ottawa-Carleton). 1999a. Trends in Water Quality Parameters Pertinent to Fish and Contaminant Levels in Fish in the Rideau and Ottawa Rivers. Prepared by the Surface Water Quality Branch, Environment and Transportation Department, Region of Ottawa-Carleton. 122 pp. (N)
RMOC (Regional Municipality of Ottawa-Carleton). 1999b. 1998 Rideau River fisheries assessment report. Index netting, nursery habitat inventory, contaminant and fall drawdown monitoring. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton, Ottawa. v + 67 pp. (authored by A. Bendig).
RMOC (Regional Municipality of Ottawa-Carleton). 2000a. Rideau River Fisheries Assessment Report. Index Netting, Nursery Habitat Inventory, Contaminant Analysis and Fall Draw Down Monitoring. 1999. Surface Water Quality Branch, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton. viii + 69 pp. (authored by S. Carroll).
RMOC (Regional Municipality of Ottawa-Carleton). 2000b. Rideau River fisheries assessment report 2000. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Surface Water Quality Branch, Environmental and Transportation Department, Regional Municipality of Ottawa-Carleton. vii + 67 pp. (authored by L. Setterington).
RMOC (Regional Municipality of Ottawa-Carleton). 2000c. Analysis of water levels, flows, and temperatures affecting fish spawning along the Rideau River. Surface Water Quality Branch, Water Environment Protection Division, Environment and Transportation Department, Regional Municipality of Ottawa-Carleton. ii + 31 pp.
Robins, C. R., R. M. Bailey, C. E. Bond, J. R. Brooker, E. A. Lachner, R. N. Lea and W. B. Scott. 1991. Common and scientific names of fishes from the United States and Canada (fifth edition). American Fisheries Society Special Publication, 20:1-183.
Robinson Consultants, Aquafor Beech, Beak International, GeoSolutions Consulting, Lloyd Phillips and Associates and Daniel Brunton Consulting Services. 2001. Carp River watershed/subwatershed study report card. Report for the City of Ottawa, Project No. 00056:iv + 99 pp., appendices A-F.
Rodd, J. A. 1912. Fish breeding. Forty-Fifth Annual Report of the Department of Marine and Fisheries 1911-12, Fisheries, Appendix No.19, Sessional Paper 22:351-368.
Rodgers, D. W. 1976. Food habits, growth, energy transformation and pollutant accumulation of yearling yellow perch (Perca flavescens, Mitchill) in the Ottawa River. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. x + 105 pp.
Rodgers, D. W. and S. U. Qadri. 1982. Growth and mercury accumulation in yearling yellow perch, Perca flavescens, in the Ottawa River, Ontario. Environmental Biology of Fishes, 7(4):377-383.
Rother, G. 1983. Management plan for sport fishing in Gatineau Park. National Capital Commission, Ottawa. Unpublished Report.
Roussel, Y. 1965. Rapport de pêche de l'éperlan à travers la glace au lac Vert, canton Hincks, comté de Gatineau. Unpublished Manuscript, 7 pp.
Rubec, P. J. 1971a. Gatineau-Ottawa Project Preliminary Report. Fish Fauna of Gatineau Park. Unpublished Report, National Capital Commission, Ottawa. 10 pp.
Rubec, P. J. 1971b. Gatineau Ottawa Project. Fish Fauna of Gatineau Park, Quebec. Unpublished Report, National Capital Commission, Ottawa. 15 pp.
Rubec, P. J. 1975a. Fish distribution in Gatineau Park, Quebec, in relation to postglacial dispersal, man's influence, and eutrophication. Canadian Field-Naturalist, 89(4):389-399.
Rubec, P. J. 1975b. Age, growth, distribution, reproductive behaviour, food habits and mercury concentrations of the brown bullhead, Ictalurus nebulosus (Le Sueur), in section of the Ottawa River near Ottawa and Hawkesbury, Canada. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. xii + 164 pp.
Rubec, P. J. and B. W. Coad. 1974. First record of the margined madtom (Noturus insignis) from Canada. Journal of the Fisheries Research Board of Canada, 31(8):1430-1431.
Rubec, P. J. and S. U. Qadri. 1977. Comparative study on age, growth and condition of brown bullhead, Ictalurus nebulosus (Le Sueur) in sections of the Ottawa River subject to industrial and municipal effluents near Ottawa and Hawkesbury, Canada. Paper presented to the Canadian Society of Environmental Biologists, Halifax (title).
Rubec, P. J. and S. U. Qadri. 1982. Comparative age, growth, and condition of brown bullhead, Ictalurus nebulosus, in sections of the Ottawa River, Canada. Canadian Field-Naturalist, 96(1):6-18.
S
Sarakinos, H., A. O. Nicholls, A. Tubert, A. Aggarwal, C. R. Margules and S. Sarkar. 2001. Area prioritization for biodiversity conservation in Québec on the basis of species distributions: a preliminary analysis. Biodiversity and Conservation, 10(9):1419-1472.
Sarsfield, T. 1967. Officials report good walleye run. The Ottawa Citizen, 4 May 1967, p. 24.
Sarsfield, T. 1974. Catch worth effort. The Ottawa Citizen, 18 February 1974, p. 13.
Sarsfield, T. 1975a. 'Unwanted' fish valuable. The Ottawa Citizen, 10 February 1975.
Sarsfield, T. 1975b. Excellent fishing spots on our doorstep. The Ottawa Citizen, 28 June 1975, p. 23.
Schueler, F. W., A. Karstad and RVCA Staff. 1996. Ecological profile, Volume 2:iv + 66 pp. In: Interim report on the functions and status of Kemptville Creek. Rideau Valley Conservation Authority. 2 volumes.
Scott, W. B. and E. J. Crossman. 1973. Freshwater Fishes of Canada. Bulletin of the Fisheries Research Board of Canada, 184:xi + 966 pp.
Scott, W. B. et E. J. Crossman. 1974. Poissons d'eau douce du Canada. Bulletin of the Fisheries Research Board of Canada, 184:xi + 1026 pp.
Séguin, R. L. 1965a. Rapport des activités et des travaux effectués au cours de l'année 1964 dans le district Outaouais-Abitibi du Service de la faune du Québec. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, unpublished manuscript. 24 pp.
Séguin, R. L. 1965b. Pêche à travers la glace au cours de l'hiver 1964 dans le district de l'Outaouais. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, unpublished manuscript. 4 pp., 2 tableaux.
Séguin, R. L. 1965c. Les espèces de poissons de la rivière Quyon, comté de Pontiac, et commentaires sur l'omble de fontaine, Salvelinus fontinalis. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, unpublished manuscript. 3 pp., 4 tableaux.
Séguin, R-L. 1967a. La pêche sous la glace au cours de l'hiver 1963-1964 dans le district de l'Outaouais. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Travaux en cours en 1964, Rapport No. 4:287-296.
Séguin, R. L. 1967b. L'omble de fontaine, Salvelinus fontinalis, et les autres poissons de la rivière Quyon, comté de Pontiac. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Travaux en cours en 1964, Rapport No. 4:5-23.
Séguin, R. L. 1970. Rapport des travaux des activités du district d'aménagement Outaouais-Abitibi pour 1965. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Travaux en cours en 1965, Rapport No. 5:199-210.
Séguin, R. L. et C. M. Veilleux. 1970. Étude de la distribution, de la reproduction et de la pêche de l'achigan à petite bouche, Micropterus dolomieui, dans le district d'aménagement Outaouais-Abitibi. Service de la Faune, Ministère du Tourisme, de la Chasse et de la Pêche, Québec, Travaux en cours en 1965, Rapport No. 5:53-68.
Selic, C. 1988. Description de certains marais de la rivière des Outaouais et évaluation par la faune. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 80 pp.
Setterington, L. 2000. Rideau River fisheries assessment report 2000. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Surface Water Quality Branch, Environmental and Transportation Department, Regional Municipality of Ottawa-Carleton. vii + 67 pp.
Setterington, L. 2002. Rideau River fisheries assessment report Eccolands Reach 2002. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Water Environment Protection Program, Environmental Programs & Technical Support Division, Utility Services Branch, City of Ottawa. v + 57 pp.
Setterington, L. 2004. Rideau River fisheries assessment summary report 1995-2000. Index netting, nursery habitat inventory, contaminant analysis, and fall drawdown monitoring. Water Environment Protection Program, Environmental Programs & Technical Support Division, Utility Services Branch, City of Ottawa. vi + 84 pp.
Sevitt, D. 2002. No need to angle for a hot spot in Ottawa because the fish are jumpin'. The Ottawa Citizen, 29 June 2002, p. D3.
Simon, T. P. and D. J. Faber. 1987. Descriptions of eggs, larvae, and early juveniles of the Iowa darter, Etheostoma exile (Girard), from Lac Heney, Quebec. Canadian Journal of Zoology, 65(5):1264-1269.
Singer, Z. 2003. Invasive Asian carp. The Ottawa Citizen, 13 March 2003, p. C3.
Small, H. B. 1883. Fishes of the Ottawa District. Ottawa Field-Naturalist Club Transactions, 4:31-49.
Small, H. B. 1893. My aquarium. The Ottawa Naturalist, 7:33-48.
Small, H. B. and W. P. Lett. 1884. Report of the Zoölogical Branch. Ottawa Field-Naturalists' Club Transactions, 5, 2(1):148-151.
Small, H. B. and W. P. Lett. 1885. Report of the Zoological Branch. Ottawa Field-Naturalists' Club Transactions, 6, 2(2):280-283.
Smith, C. H. and I. Dyck. (Eds.). 2007. William E. Logan's 1845 Survey of the Upper Ottawa Valley. History Paper 54, Mercury Series, Canadian Museum of Civilization, Ottawa. xvii + 238 pp.
Smith, N. W. 1974. Age, growth, food, fecundity, maturation and local distribution of channel catfish (Ictalurus punctatus) in the Ottawa River near Ottawa and Hull, Canada. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. viii + 96 pp.
Snetsinger, R., D. Kristensen, M. A. Snetsinger, A. Hansen and A. Blyth. 1998. A Broader View: Toward Ecosystem Management on the Rideau Canal. Report prepared by Ecological Services, Elginburg, Ontario for Parks Canada. xvii + 287 pp.
Snyder, E. 2004. Jock River stream assessment. Trail & Landscape, 38(2):71-74.
South Nation Conservation Authority. 2010. South Nation River fish community analysis 2001-2009. Fisheries Department, South Nation Conservation, Finch, Ontario. iv + 28 pp.
South Nation Conservation de la Nation Sud. 1998. Fishing Guide/Guide de pêche South Nation River/Rivière nation sud, Ontario, Canada. South Nation Conservation de la Nation Sud, Berwick, Ontario. 20 pp.
Speasr, T. 1998. Drum fish signals rebirth of Rideau: researchers find healthy newcomers in local waterways. The Ottawa Citizen, 28 August 1998, p. B1.
Spears, T. 1999a. Walleye whereabouts help monitor Jock. The Ottawa Citizen, 13 April 1999.
Spears, T. 1999b. Neighbours learn to help Rideau. The Ottawa Citizen, 13 April 1999.
Stephenson, J. M. and A. Morin. 2009. Covariation of stream community structure and biomass of algae, invertebrates and fish with forest cover at multiple spatial scales. Freshwater Biology, 54(10):2139-2154.
Stobo, W. T. 1971. Pollution and distribution and growth of yellow perch (Perca flavescens) in the Ottawa River. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario. xiii + 126 pp.
Szabo, N. 2004. Lake sturgeon in Ontario during the past century: collection and summary of newspaper articles. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 48 pp.
T
Taillefer, J. et P. Lafrance. 1971. Préparation des cartes de répartition géographique des espèces de poissons dans le district Outaouais-Abitibi. Services des études et inventaires bio-physiques, Office de planification et de développement du Québec. 39 pp. (O)
Talajic, F. 1980. Age, growth, food, sexual dimorphism, maturity, fecundity, and proximate analysis of mooneye (Hiodon tergisus, Le Sueur) in two areas of the Ottawa River. M.Sc. Thesis, University of Ottawa, Ottawa, Ontario xiii + 107 pp.
Thellen, G. 1977. Rapport sur les visites de frayères faites au printemps de 1976 dans le district de l'Outaouias. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 22 pp.
Thellen, G. 1978a. Rapport sur les visites de frayères de certaines espèces de salmonidés dans quelques plans d'eau de la région de l'Outaouias en 1978. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 7 pp. (N)
Thellen, G. 1978b. Rapport sur les frayères de doré jaune dans le district de l'Outaouias, printemps 1977. Région de l'Outaouais, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Hull, Québec. 11 pp. (O)
Thellen, G. 1994. Quelques données sur la fraye et les frayères de certaines espèces de poissons de la région de l'Outaouais. Direction régional de l'Outaouias, Service de l'aménagement et de l'exploitation de la faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 10 pp.
Thellen, G et M. Lalancette. 1992. Comparaison de la mortalité de poissons au cours de l'hiver 1987-1988 dans les baies aménagées pour la faune en bordure de la rive nord de la rivière des Outaouais entre Hull et Montebello. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 16 pp.
Thellen, G et M. Lalancette. 1994. Résultats de la pêche expérimental au lac Deschênes en 1991. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère du Loisir, de la Chasse et de la Pêche du Québec, Hull. 14 pp.
Thellen, G et M. Lalancette. 1995. Diagnose écologiques de la baie de la Pentecôte. Direction régional de l'Outaouais, Service de l'Aménagement et de l'Exploitation de la Faune, Ministère de l'Environnement et de la Faune, Hull. 29 pp., annexes.
Toner, G. C. 1943. Ecological and geographical distribution of fishes in eastern Ontario. M.A. Thesis, University of Toronto Toronto. 91 pp.
Tremblay, N. 2002. National Capital Commission LeBreton Flats Infrastructure and Remediation Project. National Capital Commission, Ottawa. 124 pp. (www.capitaleducanada.gc.ca, downloaded 29 May 2003).
Tupling, G. M. 1972. 1972 summer creel census report, Kemptville District. File Report, Ontario Ministry of Natural Resources, Kemptville, Ontario. 31 pp.
Turgeon, J. and L. Bernatchez. 2001. Mitochondrial DNA phylogeography of lake cisco (Coregonus artedi): evidence supporting extensive secondary contacts between two glacial races. Molecular Ecology, 10:987-1001.
U
Underhill, J. C. 1986. The fish fauna of the Laurentian Great Lakes, the St. Lawrence lowlands, Newfoundland and Labrador, p. 105-136. In: Hocutt, C. H. and E. O. Wiley. (Eds.). The Zoogeography of North American Freshwater Fishes. John Wiley & Sons, New York. xiii + 866 pp.
V
Vachon, J., B.F. Lavallée and F. Chapleau. 2006. Charactéristiques d'une population introduit du grand brochet, Esox lucius, dans le lac Ramsey, Parc de la Gatineau, Québec, et impact sur l'ichtyofaune. Canadian Field-Naturalist, 119(3)(2005):359-366.
Vachon, N. 2003. Identification guide and key for juvenile redhorse (genus Moxostoma) from Québec. Société de la faune et des parcs du Québec, Direction de l'aménagement de la faune de Montréal, de Laval et de la Montérégie, Longueuil, Technical Report, 16-14A:vi + 26 pp.
Vallières, L. et R. Fortin. 1988. Le grand brochet (Esox lucius) au Québec: biologie et gestion. Rapport Technique, Service de l'Aménagement et de l'Exploitation de la Faune, Québec. xv + 298 pp.
Van Cortlandt, E. 1865a. No. 1 - Minnow Lake. Notes on the lakes and lake fishes in the vicinity of Ottawa, C. W., by a member of the Isaac Walton Club. The Ottawa Citizen, 21 June 1865.
Van Cortlandt, E. 1865b. Fishes of the Ottawa. A digest of an essay on the fishes of the Ottawa River, with its tributaries and some of the contiguous lakes. - Read before the Natural History Society on Friday, 21 Nov., 1865. The Ottawa Citizen, 29 November 1865.
Van Cortlandt, E. 1867. Propagation of fish. The Ottawa Citizen, Letter dated 26 February 1867, reprinted 1 July 1999, p. A14.
Van Dusen, T. 2004. SNC cheers fish tale sighting of rare mooneye good news for waterway's health. Ottawa Sun, 9 October 2004, p. 20.
Van Dusen T. 2011. Variety of fish take a shine to South Nation. Ottawa Sun, 23 January 2011, p. 10.
Van Vliet, W. H. and S. U. Qadri. 1970. Capture, morphology and food of lake charr alevins in Lake Heney, Quebec. Canadian Field-Naturalist, 84(2):177-179.
Various Authors. 1996. Interim report on the functions and status of Kemptville Creek. Rideau Valley Conservation Authority. 2 volumes.
Veilleux, C. 1966. Evaluation du taux d'éclosion de l'éperlan, Osmerus mordax (Mitchill) au ruisseau McCloskey, comté de Gatineau, province de Québec. Thèse de maîtrise, Département de Biologie, Université d'Ottawa, Ottawa, Ontario. 36 pp.
Veilleux, C. M. et J. M. Lafrance. 1970. Etude de la reproduction du brochet Esox lucius dans la frayere Pellissier, Lac McGregor, Comté Papineau. Direction régionale de l'Outaouais, Direction générale de la faune, Ministère du Loisir, de la Chasse et de la Pêche, Gouvernement du Québec. 15 pp., 7 tabs., 6 figs.
Veilleux, C. M. et R. L. Seguin. 1968. Transformations à la frayère de brochet (Esox lucius) au ruisseau Pellissier, comté de Papineau, et observations sur les brochetons. Congrès Annuel de l'ACFAS, 8-9 novembre 1968, Ottawa, Ontario. 16 pp.
Vineberg, A. 2000. Downstream from Ottawa. The Canadian Fly Fisher, winter 2000, p. 15.
Vladykov, V. D. 1972. Lampreys of the Ottawa area. Trail & Landscape, 6(4):121-128.
Vladykov, V. D. and E. Kott. 1980. Description and key to metamorphosed specimens and ammocoetes of Petromyzontidae found in the Great Lakes region. Canadian Journal of Fisheries and Aquatic Sciences, 37(11):1616-1625.
Vladykov, V. D. and E. Kott. 1982. Correct scientific names for the least brook lamprey and the American brook lamprey (Petromyzontidae). Canadian Journal of Zoology, 60(5):856-864.
von Rosen, H. K. 1978. Creel census program summary for the Lanark, Ottawa, and Brockville districts. File Data, Ontario Ministry of Natural Resources.
von Rosen, H. K. 1989. 1989 aerial winter creel census of the Ottawa River (Cornwall and Carleton Place districts). Ontario Ministry of Natural Resources, Carleton Place, Ontario. 26 pp.
W
Wachelka, H. 1991a. Rideau River - muskellunge spawning study - Long Reach section. Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1991b. Rideau River - muskellunge spawning study - Downtown Ottawa section (Hog's Back to Brewer Park). Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1991c. Rideau River - muskellunge spawning study - Long Reach section. Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1991d. Dow's lake - City of Ottawa - fish inventory. Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1992a. Rideau River - muskellunge spawning study - Downtown Ottawa section (Hog's Back to Brewer Park). Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1992b. Muskellunge spawning study follow up - 1992- Rideau River (Downtown). Internal Report, Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario.
Wachelka, H. 1992c. Ottawa River muskellunge spawning study. Ottawa Valley Chapter, Muskies Canada Inc., Ottawa, Ontario. 6 pp.
Wachelka, H. 1996. Rideau River telemetry study, p. 67-71. In: Kerr, S. J. and C. H. Olver. (Eds.). Proceedings of Managing Muskies in the 90's. Workshop Proceedings, Kemptville College of Agricultural Technology, Kemptville, Ontario, August 16-17, 1995. iv + 170 pp.
Wachelka, H. 1999. From the chair. The Release Journal, 22(1):10.
Wachelka, H., M. Lascelles and M. Loewen. 2000. Scoping report on fish and terrestrial habitat and water quality along the Rideau River. 56 pp. (www.restoretherideau.org/resources/library.html, downloaded 16 July 2002).
Waddell, J. 1999. First muskie a monster angler won't soon forget. The Ottawa Citizen, 11 October 1999, p. B6.
Walker, J. 1905. Ottawa hatchery. Thirty-seventh Annual Report of the Department of Marine and Fisheries 1904, Fisheries, Appendix No.17, Sessional Paper 22:259-260.
Ward, B. 2004. Muskie catch makes one mean fish story. The Ottawa Citizen, 14 October 2004, p. B3.
Wooding, F. H. 1994. Lake, river and sea-run fishes of Canada. Harbour Publishing, Madeira Park, B.C. 303 pp.
Wozney, K. M., T. J. Haxton, S. Kjartanson and C. C. Wilson. 2011. Genetic assessment of lake sturgeon (Acipenser fulvescens) population structure in the Ottawa River. Environmental Biology of Fishes, 90(2):183-195.
Z
Zabel, C. 2004. Winchester, Canada. The river's full of suckers. Winchester Press (online version), 116(25), 15 September 2004, 2 pp.
Zabjek, A. 2006. A
little fish that packs a lot of science. The Ottawa Citizen, 5 August 2006, p.
E1.
© Brian W. Coad (www.briancoad.com)