Freshwater
Fishes of Iran
Species Accounts
Description
and
Petromyzontidae to Clupeidae
Revised:
10 April 2016
Back to Contents
Description
The species dealt with here in detail
have all been recorded
from Iran and confirmed by specimens. Mention is also made of other species
which occur on the borders of Iran or in drainage basins shared with Iran.
These have no valid Iranian record but may eventually be found in that
country. The listing here is selective from other papers by me on neighbouring
countries as a number of species are unlikely to enter Iranian waters because
their distributions are too remote, e.g. Cobitis elazigensis from
the Tigris-Euphrates basin at Elazig in Turkey or too restricted, e.g.
Typhlogarra widdowsoni from a cave in Iraq (see Coad, 1991b). Coad
(1995a) gives a more complete listing of species found in waters neighbouring Iran.
The most recent checklist on this fauna is by Esmaeili et al. (2010).
The definition of freshwater here includes the southern Caspian Sea
which is at one-third seawater and has both nominally marine and
freshwater species in its fauna.
The choice of introduced species to include in the Species
Accounts is somewhat arbitrary. Soviet authorities introduced a number
of species into the Caspian Sea and its tributaries and some of these became
well established, spreading to Iranian waters, e.g. Liza aurata
and Liza saliens, now commercially important. Other species did
not become established but the potential for spread was there and so they
are mentioned briefly in the Species Accounts. In northeastern Iran, the Tedzhen River flows into Turkmenistan and a number of exotic species are
known from this former Soviet republic (see Aliev et al., 1988;
Shakirova and Sukhanova, 1994; Sal'nikov, 1995). I have listed here only
ones reported from the Tedzhen River basin and its reservoirs. The Tedzhen
(Hari Rud in Iran) connects with the Karakum Canal which harbours a number
of exotics as well as species from the Amu Darya. These may be able to
colonise Iranian waters should they reach the Tedzhen River but are not
included here in the absence of definite records.
A paper in Farsi by Farid-Pak (1957) records the grayling,
Thymallus thymallus (Linnaeus, 1758), the lacustrine smelt Osmerus
eperlamus eperlamus (sic) m. sprinchus (sic)
(= Osmerus eperlanus eperlanus morpha spirinchus Pallas,
1814) and the sculpin Cottus gobio koshewnikowi Grazianov, 1907
from the Caspian coast of Iran but the first two species are distributed
in waters remote from Iran and the last has not been recorded south of
the Caucasus (Abdurakhmanov, 1962; Abbasov, 1980). They are assumed here
to be misreadings of the literature and are not included in the species list.
Some marine species penetrate the fresh waters of
southern Iran from the Persian Gulf and Sea of Oman. These species are
included in a Marine List under Checklist in the
Introduction.
They are not included in keys but more detailed descriptions of these
fishes can be found in the literature listed in the Bibliography
such as Blegvad and Løppenthin (1944), Randall et al. (1978),
Kuronoma and Abe (1986) and Assadi and Dehqani Posterudi (1997). Certain marine species do, however, spend
a significant part of their life cycle in brackish to fresh water and are given full
accounts as freshwater fishes, e.g. Carcharhinus leucas and Tenualosa ilisha.
Choice of other marine species to be given a full treatment is
dependent on frequency of capture, residence time and distance from the sea.
Coad (1991b; 2010) and the website Freshwater Fishes of Iraq
give a list of marine species known from the Tigris-Euphrates
basin but these are mostly records from the Shatt al Arab and Hawr al Hammar
in Iraq which are under tidal influence. Hussain et al. (1989) give
an account of seasonal fluctuations in species composition in the Shatt
al Arab, Iraq. Little or nothing is known of the biology of these species
in fresh and brackish waters. They are listed here to give an idea of the
diversity of species which could be found in Khuzestan and in rivers along
the Persian Gulf coast but are not covered in detail unless verified for
Iran. Al-Daham and Yousif (1990) list additional species in an Iraqi estuary
but do not distinguish the marine species which entered purely fresh water.
Taxonomy and systematics are active disciplines
and scientific names of families, genera and species recognised from Iran can
change. Older literature will be under the former name and searches for
information in such fields as ecology should take this into account. These are
described under the appropriate taxon but some significant changes, relevant to
the Iranian species only, can be simply summarised as:-
Family changes:-
Cobitidae becomes Cobitidae and Nemacheilidae (the
latter formerly Balitoridae).
Gadidae
becomes Lotidae.
Generic changes:-
Caspialosa becomes Alosa.
Barbus becomes Barbus,
Carasobarbus, Kosswigobarbus, Luciobarbus, Mesopotamichthys,
and Tor.
Chalcalburnus
becomes Alburnus.
Gobio
becomes Gobio and Romanogobio.
Leuciscus becomes Petroleuciscus and
Squalius.
Cobitis becomes
Cobitis and Sabanejewia.
Nemacheilus becomes Ilamnemacheilus, Metaschistura,
Oxynoemacheilus, Paracobitis, Paraschistura,
Seminemacheilus, and Triplophysa.
Lebias becomes Aphanius.
Neogobius becomes Babka, Chasar,
Neogobius, and Ponticola.
The Species Accounts are arranged by family after Nelson (2006).
A higher classification can be found in the
Checklist in the Introduction.
Each Species Account is comprised of the following parts:
a) Illustration
The species is illustrated by a line drawing which is accurate in respect of
body shape, number, position and shape of fins, scales and other structures.
This drawing is usually a composite one, based on both a variety of published
illustrations and on specimens.
Further illustrations are from various sources as indicated, are of varying quality and format,
and may include colour and black and white photographs.
Diagrams may also be found in the Keys to illustrate
characters not apparent in the main drawings, such as mouth structure.
b) Map
Distributions are summarized in the form of a map. Often two maps are given,
one for the whole of Iran and one zooming in on distribution if restricted to a
particular part of the country. The
maps are from a world map layer provided by Demis bv (www.demis.nl), accessed
through http://linuxgurrl.agr.ca/mapdata/itis/itisrosa.php.
Maps must be examined in conjunction with the text Distribution (see below). Map
points are are a reflection of adequately documented museum collections and
literature. As such they reflect catchability, ease of identification, rarity,
size (large species not as easily preserved in museums as small ones but perhaps
better documented, even if only in general), field work, available nets and
other equipment, contiguity to research stations and universities, road
accessibility, commercial interest, research interests, and so on. However,
while bearing all these variables in mind and reading the Distribution
summary critically, it is possible to gain a picture of fish distributions and
objective rarity of species.
Other
sources of distributional data are field notes (principally mine and those
of V. D. Vladykov) and sight and field records transmitted to me verbally
by sources judged to be authoritative.
Note that many of these localities were ascertained in pre-GPS days from maps of
varying quality and literature requiring some careful interpretation. Maps
available in the field did not always match maps examined later and once I was
lost for a whole day. Zooming in reduces accuracy proportionately.
Each symbol may represent more than one record
because of the scale of the map or because of repeated visits to the same
locality. Localities have not been sampled on a regular basis so population
trends cannot be given. The general distribution in Iran and elsewhere
is also given textually as outlined below.
The best records are those based on collections in a museum
as these can be re-examined should any questions arise about identity and
field data notes can be re-assessed for accuracy. However, the data associated
with many museum collections are too vague or too contradictory to be included
on maps with a locality symbol.
Criteria for inclusion of literature mapping records are as follows:-
1. Accurate identification (e.g. on geographical grounds;
uniqueness of species so it could not possibly be anything else; lack of
systematic/taxonomic confusion; distinctive characters cited in the text,
drawn or photographed; assessed competence of author in identification),
2. Accurate latitude-longitude data. Latitude-longitude
may be given by the author or derived by me from the literature based on
maps and gazetteers, unique locality names, and my field experience close
in time to when the material was recorded (road/river crossings have changed
in some areas with new construction after the Islamic Revolution). One
exception in accurate latitude-longitude data is that of migratory fish
- if reported from a named river then the river mouth can be recorded since
the fish pass this point on their migration (but few works mention the
extent of upriver migration so no upper limit can be deduced; when an upper
limit is given this is spot mapped; then the species is theoretically present
in a continuous distribution from mouth to upper limit
along the river but this distribution is not filled in and this presence along the
river must be assumed from the known migratory habit).
Criteria for exclusion of literature mapping records are
as follows:-
1. Generalised localities are not accepted, e.g. Safid
River is not accepted since the actual locality along this river is unknown
(except migratory fish - see above); landing ports, fish markets and fish
farms are not included as localities unless the fish capture site or release
site is known,
2. Localities with non-unique names, e.g. Hosseynabad,
a common name for many villages; Shur River, a common name for any brackish
stream, unless these have accurate qualifying data,
3. Descriptions with internal inconsistencies which cannot be resolved to one locality,
4. Named sites which cannot be found in a gazetteer; this
is often a problem with Farsi names transliterated into various European
languages with widely differing orthography,
5. Literature records which conflict with original field
notes, jar labels or catalogues unless the literature explains why it differs.
Under Sources is a partial list of material examined, most with
latitude-longitude. Some material was identified and is used in mapping
distributions but lengths were not taken and that material is not listed.
Sometimes fish were spirited away to be eaten, fell back in the river, leaped
over nets, were kept by another researcher, were seen on market stalls and the
source was given verbally, and so on. Collections in Sources may be
annotated as "no other locality data" indicating that the collection data could
not be interpreted to a latitude/longitude or was internally contradictory.
c) Scientific and Common Names
The use of scientific names is described in the
Introduction.
Scientific names are dynamic and can change as knowledge of the fishes
increases. The ones used here are the latest available.
Common names in Farsi are given with the English translation
in parentheses. Obviously some Farsi names are
merely a translation from the English common name. Note however that some
Iranian names are originally Arabic or Turkic in origin and I have not
always been able to track their meaning. Some species have no common name
and none has been advocated. Others have a common name which is applied
to all members of the same genus (e.g. nemacheilid species are called
mar mahi (= snake fish)) but this has not been repeated under each Species
Account. The common name in Russian, Arabic, Azarbaijanian, English and
from Pakistan is also given to facilitate communication and understanding;
these names are in brackets.
There are often many common "book" names for Caspian Sea
fishes. This is a result of the Russian designation of subspecies and other
categories such as natio. The names are often based on geographical locations.
These names are included here, although many of the taxa are not now recognised,
as an aid to study of the literature. The names are probably not used locally.
Azerbaijani names appear to follow mostly the Russian designations for
these subspecies and again may not be truly local names.
The names cited as by J. J. Heckel in Arabic are also
of dubious value. They are quite old, often from areas remote from Iran,
and may not be in use today. A number of common names whose origin is Arabic
are in use in Khuzestan however, although transmogrified into Farsi.
d) Systematics
An extensive synonymy or historical treatment of the mis-application
of scientific names is not given. Some earlier names can be found in synoptic
works such as Berg (1948-1949; 1949), Coad (1981d; 1985), Krupp (1985)
and others. In certain cases, systematic or nomenclatorial problems remain
unresolved and these are briefly discussed.
Type locality is given for species originally described
from Iran or immediately adjacent waters. This type locality is given as
cited in the original text description in quotes ("....") wherever possible.
Some type localities are not given in quotes, e.g. middle Caspian Sea,
to denote they are a general indication of where the fish was first described
- this is usually applied for older literature not at hand or for fishes
not described from Iran but nearby waters. The original text, jar labels
or catalogues may be compared and interpreted where these are unclear,
contradictory or spellings of place names have changed markedly. Most agree
well between these three sources and are easily located with due allowance
for variant spellings, handwriting skills and transcription errors. Disposition,
number and condition of types may vary with time however. Eschmeyer's on-line
"Catalog of Fishes" has disposition of types but these records are
only as good as the most recent revision of the taxon concerned. Latitude
and longitude are calculated for type localities in Iran wherever possible.
Note that transliteration from Russian names often gives
variant spellings for authors of species names. Actual dates of publication
may vary one or more years subsequent to the date on the journal or article,
i.e. publication may be delayed. This may not be evident from an examination
of the article but may be known to the author or others familiar with the
situation. This has not always been clearly set down in print and accounts
for varying publication dates in different sources.
The disposition and condition of type material is given
where known along with catalogue numbers. Museum acronyms are from Leviton
et al. (1985) but these may change, notably ZIL (Zoological Institute,
Leningrad, U.S.S.R.) became ZISP (Zoological Institute, St. Petersburg,
Russia) and the British Museum (Natural History), London became the Natural
History Museum but retained BM(NH) as its acronym. Note that knowledge
of type material in museums changes as the specimens are examined over
time. Not all new information is published as it is the result of in-house
curatorial work and may only be available in catalogues and jar labels.
The information cited here is the most recent available to me.
Subspecies and lower, non-taxonomic categories have received
names. Such taxa (and non-taxa) have a narrower range of meristic characters and
certain distinguishing other characters compared with the species. Ranges and
descriptions apply to the species as a whole, since many subspecies appear to be
ill-founded where they have been studied in more detail, and indeed some species are not distinct but members
of a wide-ranging and variable species. Certain subspecies may be valid,
or their status is undetermined by recent study, and characters for these
are given separately, either here or in Key characters or Morphology.
e) Key characters
The characters detailed here will separate the species
from any Iranian freshwater fish. These characters (and the keys) should
not be used to identify species from countries bordering Iran as they are
specific to Iran.
f) Morphology
Under this heading are described a number of features
which add to the key characters in describing the fish. Morphometric characters
are not often used since the shape of body parts can be seen in the drawing
and such characters vary greatly with sex and size in contrast to meristic
characters. The accurate explication of morphometric characters depends
on comparative statistics and is beyond the scope of this work. The assessment
of variation between adults and juveniles or between geographical localities
is limited by material and its presentation here by space.
The chief characters summarised here are meristic or countable
characters. These include counts of scale, fin rays, vertebrae, gill rakers,
and teeth. They are summarised as ranges based on literature sources (including
my own data where this expands ranges). In certain cases literature data
is extensive and swamps the few specimens available from Iranian waters.
The literature ranges give an indication of how variable a species may
be in a given character; data on a few Iranian specimens would give a misleading
picture of potential variation which future students of Iranian fishes
may find. Counts from Iranian specimens made by me are given with frequency
in parentheses, e.g. dorsal fin branched rays 7(3), 8(34), 9(5) indicates
that 3 fish had 7 branched dorsal fin rays, 34 fish had 8 branched rays
and 5 fish had 9 branched rays.
g) Sexual dimorphism
Males and females often differ markedly in appearance,
whether in colour, body proportions or in structural features and these
are detailed here to obviate misidentifications.
h) Colour
The colour patterns of fresh and preserved specimens including
males and females, young and adult, and spawning and non-spawning individuals
are given where known. Colour can be a key character in determining the
species but is also variable and should be treated with care in identifications.
Some fish change colour to match their background or pale in response to
a threat. Fish from muddy waters in Iran are often washed out and greyish
in colour. Immersion in ice water enhances the colour patterns and some
of this is retained in preservative.
i) Size
The maximum reported size is recorded as total length
or standard length (if not specified then the source did not indicate which
length was measured) and weight where known. These measures are not restricted
to Iranian specimens since sample sizes are small for some species and
would give a false picture of maximum size.
j) Distribution
This section summarises distribution for the whole range
of the species both within Iran and the rest of the world. Within Iran
the general distribution is given. The detailed mapped distributions are
based on collections or literature with adequate data (see above under Map). Some literature
and museum records are given simply as, e.g. "Safid River", which cannot
be mapped accurately but can be cited in this section. Some literature records
are included here but not every locality based on my field collections as these
are summarised on maps. Not every river
mentioned in the literature is listed here, as common species are assumed to be
widely distributed within a basin; generally only those major rivers or general
localities that are in basins without a mapped distribution are cited.
k) Zoogeography
The relationships of the species, its origins and movements
in the past are given here, where this has been determined.
l) Habitat
The type of habitat favoured by the species is outlined
and includes such factors as altitude, substrate, temperature, salinity,
oxygen, flow regime, pH, vegetation, turbidity, pollution resistance, etc.
There are few detailed studies of habitat requirements for many species:
some can be deduced from morphology. Field data can give a partial picture
but are often limited to one time measurements of seasonal and daily variables
such as temperature which are necessarily of restricted value.
Colour illustrations of habitats are included where available.
m) Age and growth
This section, and the following two sections, either have
no information or masses of information. The Caspian Sea basin species
are often widely known and have books and numerous papers written about
them. There is also a vast "Soviet" literature on some of these species
but I did not have the time nor the resources to digest it all. Here only brief
summaries can be given and it is not always clear whether the Iranian populations,
often at the southern edge of the species range, or recognised as a distinct
subspecies, have the same general ecology as European or more northerly
"Soviet" populations.
Most species outside the Caspian basin are poorly known
ecologically. I have attempted to summarize what is known based on literature
in particular from Iraq and Turkey where ecological studies of varying
quality have been published on some of the species. Morphology can be used
to gain a general picture and knowledge of related species helps.
Generally growth in fishes is fastest in the youngest
age groups, slowing with age and with investment in reproduction. Maximum
age varies considerably, some small species living only a few years while
others are much larger and are reputed to live longer than people. Conventionally,
age may be represented by a number then the + sign, e.g. 0+ = a fish in
its first year of life, less than one year old; 6+ = a fish between 6 and 7 years old.
n) Food
Diet is reported from literature studies and from brief
examination of gut contents by me. Diet varies seasonally, daily, with
age, between sexes, and with changes in environmental conditions but most
fish concentrate on one or a few major groups. These are scrapers, invertebrates
and fishes, and rarely aquatic macrophytes.
o) Reproduction
The spawning season, migrations, egg numbers and diameters, and reproductive
behaviours are recorded here. Some migratory behaviour and ages at spawning may
be recorded in the the Habitats and Age and growth sections.
p) Parasites and predators
This section contains information on the parasites and
predators of the species described. I have recorded only parasites known
from Iranian populations. There is a more extensive literature on Iraqi
populations (see Mhaisen, 1980; Coad and Al-Hassan, 1989) and on European
or Caspian Sea populations (see Romanov, 1955) for species found in Iran.
For eastern waters consult Moravec and Amin (1978) on Afghanistan and Mirza
(1978) on Pakistan. In the absence of definite records for Iran and in
the interests of saving space, I have not cited this extensive literature.
Pazooki and Masoumian (2012) provide a synopsis of parasites in Iranian
freshwater fishes and this extensive list (247 species) has not been integrated
into the text of the present work.
There are a number of piscivorous birds in Iran (see Scott
et al. (1975), Behrouzirad (2007) and general field guides) and these take fishes but
there seems to be little direct observation on the fish species preferred.
q) Economic importance
Note that fishery information may be given on an annual
basis but the year reads 1965-1966 or 1965/66; Iranian years start in March
and run across 2 western calendar years.
r) Conservation
This section details conservation measures undertaken or needed for the species. A
general survey of conservation status of native Iranian freshwater fishes is
given by Coad (2000a).
s) Further work
This section gives some suggestions for knowledge gaps that should be filled.
t) Sources
This section refers to papers or synoptic
works on the species in addition to those cited in the text. It
should be noted that a number of synoptic works refer to several species
in Iran, e.g. Berg's "Freshwater Fishes of the U.S.S.R. and adjacent countries",
and these are not listed repetitively under each Species Account although
they are to be found in the Bibliography. Web sites or URLs are cited as
documentation of statements but it should be noted that these may become broken
links and they are not continually verified as active.
Descriptions are based on Iranian specimens wherever possible but additional
material from neighbouring countries has also been examined. Meristic counts,
for example, are given as frequency distributions for Iranian material while general
ranges for these characters are based on Iranian material, on literature and on
counts of other specimens listed here briefly. Descriptions are also based on
material seen in bazaars or captured in the field but not retained, and on
photographs, drawings, field notes of other collectors, and verbal descriptions
of other scientists.
Details on collections are on file at the Canadian Museum of Nature, Ottawa and in other
institutions as recognised by their acronyms. Locality data is given in short form
and the reader is referred to the website of the relevant museum for further information.
Locality names are taken from U.S. Board on Geographic Names publications and
these may vary from names on labels in museums. The Board names contain both
conventional and local Farsi, Arabic and Turkish names of localities. I have
interpreted names as best I can and have, for example, retained English names
for major water bodies and towns where a strict usage would be bewildering, e.g.
Harirud = Tedzhen River, Sefidrud = Safid River, Al Mawsil = Mosul, Darya-ye
Mazandaran = Caspian Sea, and so on. Sometimes a collection is annotated as "no other locality data", indicating that
no further details are known or localities cited could not be found on maps or
in a gazetteer (and thus there is no latitude-longitude). Collections listed as uncatalogued are mostly held in the Canadian Museum of Nature and may eventually
receive a catalogue number. The collections listed are those examined for morphology.
Map records include these collections, other collections checked for identity and
locality only, and literature sources, all kept in a database held at the Canadian
Museum of Nature: these would be too lengthy to list here.
Petromyzontidae
Back to Contents
Lampreys in the family Petromyzontidae are found in cooler waters of the northern hemisphere,
with a few related species in other families in the southern hemisphere.
Their origins lie at least 300 million years in the past.
There are about 43 lamprey species in 9 genera (Eschmeyer and Fong, 2011) with only 1 recorded from Iran.
Lampreys are jawless fishes, lacking bone in the skeleton and
having 7 pairs of pore-like gill openings. The eel-like body has no
pectoral or pelvic fins. There are 1 or 2 dorsal fins and a caudal
fin. An anal fin-like fold develops in spawning females. The mouth is
a suctorial disc armed with rows of horny teeth. There are also teeth
on the tongue. The median nostril, or nasohypophyseal opening, is not
connected to the mouth. There is a light-sensitive pineal organ or
"third eye" behind the nostril. The skin is covered in mucus
which is poisonous to fishes and humans. Lampreys are edible if the
mucus is cleaned off.
Their tooth arrangement is used in classification and
identification along with the number of myomeres (muscle blocks along
the body). Both tooth counts and the number of cusps are used, in
particular those on the supraoral lamina (bar above the
"mouth", the oesophageal opening), the infraoral lamina (bar
below the "mouth") and the row of teeth on both sides of the
"mouth". There are various series of smaller teeth and of
course teeth on the tongue. Larval lampreys lack teeth and are
particularly difficult to identify and their determination often
requires specialist knowledge. Characters for the larvae include
counts of myomeres and pigmentation patterns.
Lampreys have an unusual life cycle. Adults die after spawning and
the eggs develop into a larva, known as an ammocoete, which lacks
teeth, has an oral hood, eyes covered by skin, a light-sensitive area
near the tail, and is a filter-feeder while buried in mud and silt.
Fleshy tentacles in the oral hood are used to extract minute organisms
from the water, such as algae (desmids and diatoms) and protozoans.
After several years (up to 19 but usually 7 or less), the ammocoete
transforms into an adult with enlarged eyes, teeth, a different colour
and pronounced dorsal fins. The body shrinks during this metamorphosis
and adults are only larger than ammocoetes if they feed. The adult may
be a parasite on other fishes and marine mammals, or non-feeding.
Individuals of a species may or may not be parasitic and different
species may be parasitic or non-parasitic. The non-parasitic species
are believed to have evolved from a parasitic species so there tends
to be closely related parasitic/non-parasitic species pairs.
Parasitic adults feed mostly on other fishes, attaching to their
bodies by suction and using their toothed tongue to rasp through the
skin and scales to take blood and tissue fragments. Prey is detected
by sight but some lampreys attach to hosts during the night. Perhaps
this reduces their own predation risks and enables them to approach
their quiescent hosts more easily. Lampreys tend to select larger fish
as these survive longer and ensure a good food supply. The flow of
blood is aided by an anti-coagulant in lamprey saliva called
lamphedrin which also serves to break down muscle tissue. The attack
may weaken or even kill the host. Weakened fishes are more prone to
diseases and the wound provides an easy path of entry for them. The
fish (and marine mammal) species parasitised are varied and reflect
availability in the habitat.
Marine lampreys enter fresh water to spawn and freshwater species
may move into or up streams. The scientific name of the family means
"stone sucker" and the adult mouth is used to hold or suck
onto stones as well as on prey. This suction enables the lamprey to
maintain position in fast-flowing streams when spawning and even to
climb over rapids and small waterfalls. Usually spawning occurs in
shallow water with a moderate current, a bottom of gravel and nearby
sand and silt for the ammocoetes to live in. Either or both sexes
build a nest by moving gravel around with their sucking mouths and by
thrashing their bodies. A shallow depression is formed, about 0.5-1.0
metre long. Spawning often occurs in groups and several males may
attach to a female with the sucking disc. The process takes several
days as only a few white to yellow eggs are laid at a time. The eggs
are adhesive.
Adult lampreys are usually caught when attached to a host or when
spawning. Electro-shocking will force ammocoetes out of their u-shaped
burrows to the surface and immobilize adults. They sometimes attach to
boats and occasionally to human swimmers when their skin is cool but are
easily removed, perhaps because nobody has left a lamprey on their
skin long enough to see if the tongue starts rasping flesh!
Genus Caspiomyzon
Berg, 1906
This genus is characterised by having 2 dorsal fins, an oral disc
narrower than the body, teeth are generally low and blunt, the
supraoral lamina is small, oval and sometimes has 2 tubercles and
rarely 2 teeth, the infraoral lamina has 4-6, usually 5, teeth which
may be bicuspid at their tips, there are about 8 small teeth of equal
size in the transverse lingual lamina, the exolaterals, anterials and
posterials are strong and close together, anterior and endolateral
circumorals 9-11, usually 11, and 3 long, papillose velar tentacles are present.
The first illustration below shows a notch at the end of the second dorsal fin
which is an error.
There is a single species in the genus found only in the Caspian
Sea basin. Agnathomyzon Gratzianow, 1906 and its subgenus Haploglossa
Gratzianow, 1906 are synonyms of Caspiomyzon (Eschmeyer et al., 1996).
Caspiomyzon wagneri
(Kessler, 1870)

Adult
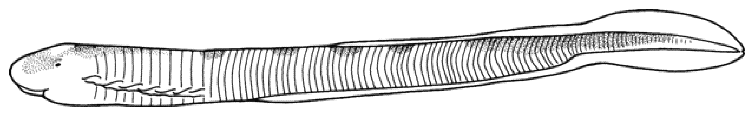
Ammocoete

Disc

Adult, courtesy of Afshin Afzali, University of Tehran

Adult, Shirud River, courtesy H. Nazari

Siardarvishan River, April 2010,
courtesy of K. Abbasi

Adults, Shirud River, courtesy H. Nazari.

Disc, courtesy H. Nazari

Disc, Siardarvishan River, April 2010,
courtesy of K. Abbasi
Common names
مارماهي (= mar mahi, meaning snake fish),
مارماهي دهان گرد
(= marmahi-ye dehangerd, meaning round mouth snake fish), mahi dehangerd, mahi dehangerd daryacheh-ye
khazar or dahangerd-e-Daryaye Khazar (= Caspian Sea round mouth fish).
[ilanbaligi or xazar ilanbaligi, djilan-balux or morma in Azerbaijan; kaspiiskaya minoga or Caspian lamprey in Russian; Volga lamprey].
Systematics
The type locality of Petromyzon Wagneri is from the mouth of
the Tvertsa to Astrakhan; Oka and Kama rivers and the 3 syntypes
(29.0-33.0 cm) are in the Zoological Institute, St. Petersburg (ZISP
31) (Holčík, 1986). The Zoological Museum of Moscow University (ZMMU) has one syntype from
the Kura River near Evlakh (P-1393) and one from the Moskva River (P-555) with
P-569 from the Volga River near Kazan being lost (Pavlinov and Borissenko,
2001). The Naturhistorisches Museum Wien in 1997 had one specimen
listed as "? syntype, ? paratype" (sic) under NMW
61053. Agnathomyzon (Haploglossa) caspicus Gratzianow, 1907 is a synonym.
Key characters
This is the only lamprey species in Iran, easily recognised by the
absence of pectoral and pelvic fins, a round, suctorial mouth
containing blunt teeth, and 7 branchial openings.
Morphology
Characters of the species are the same as the genus. Trunk myomeres
number 53-68 in ammocoetes; and 68(2) or 69(1) in adults from Iran. Ginzburg
(1936a) describes ammocoetes from Iran. Renaud et al. (2009) give details
of the feeding apparatus. Nazari et al. (2009) found significant
differences for morphometric, but not meristic, characters, between fish from
the Shirud and Talar River, although a principal components analysis showed
relatively high overlap.
Renaud (2011) gives details of morphology.
Sexual dimorphism
Females reach larger sizes than males and have a smaller urogenital
papilla. During the spawning migration, the lamprey undergoes certain
morphological changes some of which have been linked to sex of the
fish. The teeth become blunt, fin size increases, the dorsal fins
become almost united at the base in males, and there is a change in
colour. The urogenital papilla length in males increases from a mean
of 1.1 mm to 4.9 mm and in females from a mean of 0.6 to 1.7 mm.
Colour
Adults are dark grey with a silvery-white belly. Spawning adults
become black on the back and flanks with a grey belly covered with
dark oval spots, or are an overall golden colour (Hassan Nazari, pers. comm., 28
July2011, see photo above). Ammocoetes are a pale grey to yellowish with a white belly.
Size
Attains 57.5 cm total length and 205.5 g as the adult and 13.0 cm total
length as the ammocoete. After metamorphosis of the ammocoete there is a shrinkage
in length, the difference between prespawning and spawning adults
being on average 22.3% in Iranian samples (Renaud, 1982). There is
also a small variety which measures 19-31 cm and can attain sexual
maturity at 19.1 cm (forma praecox).
Distribution
Found only in the Caspian Sea and rivers draining to it, in
particular the Volga where it had its largest distribution but is now
known only as far as the Volgograd Reservoir; also in the Ural, Terek,
and Kura rivers. It is recorded in Iran from the upper reaches of the Aras River,
and from the Astara to the Gorgan River along the whole Caspian coast. Specific
localities include the Aras River, Anzali Mordab and the Nahang Roga, Pir Bazar Roga,
Pasikhan River and Siah Darvishan River in the Anzali region, to Kisom on the
Safid River, Cheshmkelya east of the Safid River, Tajan River, Sardab River, Haraz River,
Babol River, Tonekabon River, Pol-e Rud, Gorgan River, and in most
large streams (Derzhavin, 1934; Holčík and Oláh, 1992; Hosseinpour, 1995;
Abbasi et al., 1999; Kiabi et al.,
1999; Abdoli, 2000; Abdoli and Naderi, 2009). Migrations into the Babol, Gorgan and Sardab rivers are
reported by Ghasempouri (1993), the Sardab and Chalus rivers by the Annual
Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran
(1996), and the Shirud (Nazari and Abdoli, 2010), for example.
Zoogeography
Known only from the Caspian Sea, its relationships remain uncertain and
research is ongoing (Claude B. Renaud, pers. comm., 18 May 2007).
Habitat
The habitat of this species in the southern Caspian Sea proper is unknown
although some specimens have been caught in the Caspian at 600-700 m (Jolodar
and Abdoli, 2004). Larvae burrow 1-2 cm into the river bottom and favour areas where
current is moderate at river bends. They can also be found in the
centre of rivers or in backwaters. Fine-grained sand with some ooze
and detritus is preferred at all stages of larval growth but larger
larvae can also be found in a silt-sand bottom with much plant debris
and macrophytes. The ammocoetes select and change habitat according to
sediment size as they grow. They prefer depths greater than 3 m as
protection against drying out, are mostly shallower than 11 m but as
deep as 22 m (Ginzburg, 1970), yet in different rivers or at different
times will be concentrated in water of markedly different depths, e.g.
30-85 cm versus 6-8 m.
Spawning migrations up the Volga River used to exceed 1500 km but
construction of dams now prevents this. The lamprey migrates in
schools with the smaller fish arriving in estuaries first. Larger
lampreys migrate more quickly and travel further. The speed varies
from 1.9 to 15.9 km/day. The migration is triggered by decreasing
water temperature and increasing water level. The strongest migration
is reported at 6-11°C. Movement upriver only occurs at night, near the surface when dark and
on the bottom when the moon is out. During the day, the lampreys hide
among stones. Body fat in the Volga delta was 34% but by the time the
fish reached the spawning grounds upriver it had declined to 1-2%. In
the Kura River of Azerbaijan, the lamprey migrates at the same time as
the Caspian salmon (mahi azad, Salmo caspius) and often attaches to the opercular region of this
species. The peak of this run is in December and January. The
migration in the Volga takes place from the middle of September to the
end of December. Migrating lampreys prefer a current velocity of
0.4-0.6 m/sec and stay close to banks and the bottom. Prespawning
adults overwinter among stones or in the substrate of rivers.
During winter-spring several individuals may be found coiled in a ball under
stones (Askerov et al., 2001). They hardly respond to external stimuli
such as noise or being handled. Transformed lampreys migrate to the Caspian Sea.
Before breeding, males change colour, increase slightly in size, develop their
fins, and become much more active (Askerov et al., 2001).
Nazari and
Abdoli (2010) note a short fall migration in late September to October with the
main migration being in spring (see below). Movement was mostly at night and
involved swimming and resting attached to the concrete of a bridge used as the
observation post.
Age and growth
The growth rates of metamorphosing lampreys and adults are almost
unknown. Length and weight decrease but coefficient of condition
increases in spawning as opposed to pre-spawning adults. The shrinkage
in mean total length is 18-26%. Females are heavier than males up to
about 43 cm but past this point males weigh more. There are 3 age
groups of larvae in the Volga (Ginzburg, 1970), with average lengths
of 3.1 cm, 6.2 cm and 10.1 cm and 2-4 age groups in the Kura. In their
fourth year of life they metamorphose to adults after a downstream
migration into the Caspian Sea. Adult life span is at least 1 year and
5 months. Maturity is attained in May and the beginning of June in the
Volga, and from May to the end of July in the Kura River. Mature
lampreys are mostly 35-41 cm in the Volga and 41-46 in the Kura River.
The female lamprey dies after spawning but the male may live longer
until sperm production ceases.
Nazari et al. (2010) investigated growth parameters in fish from the
Shirud and Talar River. Most fish were were in the 367-369 mm length group,
length-weight relationship was positive, high and significant, growth was
negatively allometric, the coefficient of condition was higher in females, sex
ratio was nearly equal, and growth parameters were similar in the two rivers.
Food
Abakumov (1959) maintains that this lamprey attacks Caspian salmon
(Salmo caspius) based on nineteenth century observations
by Kessler (1870a) and Kavraiskii (1896-1897). Lelek (1987) also
considers it to be parasitic. The lampreys may only have been using
Caspian salmon for transport. Certainly the teeth in this lamprey are
blunt, unlike those in lamprey species known to parasitise fishes. In
contrast, Holčík (1986) states that it is non-parasitic and Ghasempouri (1993) agrees.
Renaud (1982) supposes that adults feed on amphipods since juvenile
acanthocephalans (Corynosoma sp.) are found in prespawners.
This worm has amphipods as the intermediate host. However, Holčík (1986) thinks that
the acanthocephalans are swallowed while the adult
lampreys are feeding on the internal organs of dead fish they
scavenge. Certainly larvae of Corynosoma strumosum (perhaps
correctly C. caspicum: B. Kiabi, in litt., 1994) are
found only in the body cavity of fishes. Renaud et al. (2009) list it as
a carrion feeder but note the well-developed buccal glands which may compensate
for the blunt teeth and it may well feed on fishes. The feeding habits of the
adult of this species remain to be confirmed by direct observation. Gut contents include
aquatic vegetation in Iran and in the Volga delta. Migratory,
transforming and spawning lampreys do not feed. The gut diameter
decreases from 2.7 mm in prespawners to 1.4 mm in spawners in Iran (Renaud,
1982). Ammocoetes feed on detritus and diatoms.
Reproduction
Ginzburg (1969; 1970) examined the reproduction of this species
below the Volgograd Dam on the Volga River and similar conditions may
obtain in Iran. The dam has probably increased fecundity by reducing
the length of the spawning migration so that the fish have more energy
reserves for egg production. A spawning migration exists from December
to May with a peak concentration in the second 10 days of February
although the catches declined in April at least in part because of the
opening of the spillway of the dam. Before the dam was built the
migration from the Caspian Sea passed through the delta from
mid-October to mid-December, with a peak in December. The fish
migrated when water temperatures reached 10-11°C
and moved through channels where the current was strongest. Spawning
begins at 15-16°C, usually in early June but sometimes at the end of March through to the
beginning of July, and temperatures during spawning are usually 15-23°C.
Each female produces up to 60,000 turquoise or blue-green eggs and
spawns once in her lifetime. Eggs are ovate and diameter reaches 1.5
mm. The eggs are laid on coarse to fine-grained, turquoise sand at a
water depth of 3.5-19.0 m, sometimes shallower. The egg colour is
cryptic against the sand substrate. Many eggs are carried downstream
by the current. A redd is excavated in sand or gravel by the male or
by the female (authors differ on this point) and the lamprey attaches
to stones by their suctorial disc. The male attaches to the female's
head with his disc and wraps his body around hers. The tails of both
fish quiver and eggs and sperm are released at the same time. Females
release all their eggs but males may spawn again with other females.
Ammocoetes hatch after 8-10 days at 17-23°C.
Metamorphosis of ammocoetes occurs at 8.0-11.0 cm in October in Iran.
Nazari and Abdoli (2010) examined migration and reproduction in lampreys from
the Shirud in the southern Caspian Sea from 16 March to 2 May at 11.0-21.25°C.
The most intensive migration was at night (peaking at 2100 and declining to 0300
hours) at 16°C (34.4% of the run). About 75% of the run had passed by the
time water temperature reached 16-17°C. Migration stopped when temperature
reached 21°C. Numbers observed each night varied from 1 to 60, average 17, with
peak migrations at 26 March to 10 April and 15 April to 25 April. Sex ratio was
1.07:1 in favour of males but not significantly different. Absolute fecundity
was 31,758-51,198 eggs (mean 41,924 eggs) relative fecundity was 80.3-148.1
eggs/mm length (mean 107.2 eggs/mm length) and 260.8-677.4 eggs/g (mean 397.6
eggs/g). Egg diameter was 0.78-1.15 mm (mean 0.92 mm). The gonadosomatic index
of females was 5.83-31.44 (mean 11.22), the peak being in mid-April. Downstream
migrating lampreys were spent but no dead ones were noted so some may survive to
spawn a second time. Two
ammocoetes, 20 and 22 mm long, were found near the mouth of the Shirud River on
18 April 2006 (river bank in a substrate of the sand-mud, water depth <30 cm).
They probably belong to the autumn migratory group (Hassan Nazari, pers.
comm., 28 July2011).
Ahmadi et al. (2011, 2011) also examined fish from the Shirud and found both fall and spring migrants ready for spawning with no
differences in weight, length, absolute fecundity (17,778 eggs in spring, 20,247
in fall - see above study), egg diameter (800 μm in spring, 710μm in fall) and
sex ratio (close to 1:1). The gonadosomatic and hepatosomatic indices were
higher in fall females. Fall migratory fish had a lower condition factor.
Parasites and predators
See above under Food. Nazari et al. (2010) also record
Corynosoma in their fish. Caspian lampreys are eaten by Silurus
glanis, Lota lota, Sander lucioperca, and Huso huso.
Economic importance
This species was consumed and used for oil extraction in the former
U.S.S.R. (Thomas, 1961; Ginzburg, 1969). Their fat content is so high that they
were once dried and used as candles (Kottelat and Freyhof, 2007) and the high
fat level makes them tasty (Askerov et al., 2001). The catch in the
Volga-Caspian region was 3,420,000 kg or 33.4 million fish in 1913 but
fishing by state organizations ceased after the Volgograd reservoir
was constructed. The mean annual catch in Azerbaijan for 1930-1963
ranged from 10 to 269 tonnes. Local fisheries continue but are of
little significance. It is not commercially important in Iran for
religious reasons but catches of several hundred kilograms can be made
in an hour in such rivers as the Gorgan, Babol and Sardab (Ghasempouri, 1993).
This lamprey is ingested medicinally for treatment of haemorrhoids and besmi
(sic, ?) by Turkmen of the southeastern Caspian (Hassan Nazari, pers.
comm, 29 July 2011).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, in textbooks and
because it is reputedly ichthyosarcotoxic. Intoxication results from
eating the flesh, skin or surface mucus of raw or cooked Caspian
lamprey, the location of the toxin being uncertain. A biogenic amine
is believed to be responsible. Mucus may cause skin irritations.
Poisoning can be avoided by soaking the lamprey in brine as cooking
alone is insufficient. Symptoms develop in a few hours and include
nausea, vomiting, dysenteric diarrhoea, urge to urinate or defecate
without ability to do so, abdominal pain and weakness. Recovery takes
several days and treatment is symptomatic (Coad, 1979b). However
lampreys lack scales and are not eaten in Iran.
Conservation
The Caspian lamprey has been proposed for inclusion in the
"Red Book of the U.S.S.R." which forms the basis for
measures to protect species (Pavlov et al., 1985) and is listed
as "vulnerable" in Europe by Lelek (1987) and Maitland
(1991). It is vulnerable because it migrates into rivers which are
polluted and dammed and because of its restricted and declining
distribution. These conditions apply particularly in Iran, although
there is some evidence for spawning based on captures in the 1990s (Holčík and Oláh, 1992).
Kiabi et al. (1999) consider this species to be near
threatened in the south Caspian Sea basin according to IUCN criteria.
Criteria include medium numbers, habitat destruction, widespread range
(75% of water bodies), absent in other water bodies in Iran, and
absent outside the Caspian Sea basin. Mostafavi (2007) lists it as near
threatened in the Talar River, Mazandaran.
Further work
The question of adult diet remains unresolved and the general
biology of this species in Iran needs to be elucidated.
Sources
The main source of information on this species is the summary by Holčík (1986)
which should be consulted for further details on morphology and biology.
Iranian material: CMNFI 1970-0511, 7 ammocoetes, ? 30-82 mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0514, 33
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0515, 18
ammocoetes, ? 25-98 mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0534, 30
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0535, 14
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0545, 1
adult? see photo?, ?mm total length, Gilan, Safid River (37º01'N, 49º38'E); CMNFI 1970-0546, 2 adults,
352.0-355.0 mm total length, Gilan, Safid River (no other locality data); CMNFI 1970-0547,6
adults and 2 ammocoetes, ? photos? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0585, 3
adults, 406.0-455.0 mm total length, Gilan, Nahang Roga River (37º28'N, 49º28'E); CMNFI 1971-0327A, 1
adult (part of trunk), Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI
1979-0787, 11 adults, ?mm total length, Gilan, Nahang Roga River (37º28'N, 49º28'E); CMNFI 1980-0118,8
adults, ? mm total length, Gilan, Gelroudkhan River, tributary of the Anzali Mordab (no other
locality data); CMNFI 1980-0119, 10 adults, ? mm total length, Gilan, Gelroudkhan River, tributary of the Anzali Mordab (no other
locality data); CMNFI 1980-0139, 44 ammocoetes, ? mm total length, Gilan, Golshan River estuary (37º26'N, 49º40'E).
Acipenseridae
Back to
Contents
The family is found in Europe, northern Asia and North America with
4 genera and 26 species (Eschmeyer and
Fong, 2011). The Caspian Sea basin contains 2 genera and 6
native species, with both genera and 5 species recorded from Iran. The
Caspian population of sturgeons is the largest in the world (Levin,
1997) and Iran is the world's second largest producer of this resource
after the former U.S.S.R. (Josupeit, 1994).
These very large fishes are characterised by 5 longitudinal rows of
well-developed, bony plates along the body. There is a dorsal row, a
lateral row on each side and a ventro-lateral row on each side. In
young fish these plates are sharp and obvious but they become smoother
with age and may disappear completely. The unpaired fins have fulcra,
or flat bony plates, distinct from the scutes, in front of them. Small
plates, grains and denticles cover the remainder of the body and the
head is covered by large bony plates. Sturgeons have an elongate
snout, an inferior protrusible mouth without teeth in adults, fleshy
lips and 4 barbels in a row in front of the mouth (see Keys). The
vertebral column turns upward at the end into the upper lobe of the
tail (known as a heterocercal tail). The first pectoral ray is a
strong spine. There are few gill rakers under a single large gill
cover. The skeleton is cartilaginous, there is a spiral intestinal
valve, 1 branchiostegal ray, fin rays number more than the underlying
basal bones which support them, no gular bones on the lower head
surface and a large swimbladder. The karyotype may be complex with a
very large number of chromosomes, including the very small
microchromosomes, and tetraploidy, e.g. Huso huso, Acipenser
nudiventris and A. stellatus have 2n about 120 while A.
gueldenstaedtii has 2n about 240 and is a tetraploid. Karyotypes
of 120 chromosome species are very similar indicating a slow
evolution, correlated with a slow rate of DNA and protein evolution.
Hybridization is common, even between genera, and hybrids are fertile
and used in aquaculture in Russia (Birstein, 1993). Artyukhin (1995)
gives a phylogenetic tree of Acipenser and Huso. Krieger et al.
(2008) reviewed the molecular phylogeny of the order Acipenseriformes and found
Huso not to be monophyletic, among other unusual placements. They
conclude that some revision of classification may be needed. Rastorguev et al.
(2008) examined mtDNA for Ponto-Caspian sturgeons, although sample sizes were
small, and determined various relationships; Huso was basal with Atlantic
species and all species in the gueldenstaedtii complex were closely
related.
A
general overview of sturgeon systematics and biology is given by
Williot et al. (1991) and Billard (2002). Artyukhin (2006) and Peng et al. (2007)
summarise the relationships aned biogeography of major clades for the order (Acipenseriformes)
which dates back 200 MYA to at least the early Jurassic. A bibliography of sturgeons can be found
at www.geocities.com/CapeCanaveral/Hall/1345/sturgbibl.html.
Sturgeons are subject to overexploitation, a problem addressed by
Lukyanenko (1992), Vadrot (1990), Bemis and Findeis (1994), Faber
(1994), Moghim (1994), Anonymous (1995), Asadollahi (1995), Ivanov et
al. (1995; 1995, 1999), Vlasenko (1995), Waldman (1995), Birstein
(1996), Emadi (1996a; 1996b), DeSalle and Birstein (1996), Hosseinie (1996),
Khodorevskaya et al. (1997), Matthews (1998), Khodorevskaya and Krasikov (1999),
G. Strieker (in CNN.com, downloaded 9 March 2002), Speer et al. (2000),
Raymakers (2002), Oliver (2003), Harrison (2005), Pourkazemi (2006b), Karayev
(2006), Raymakers (2006), and numerous newspaper and
magazine articles. The problems for sturgeon survival in the Caspian
Sea and other waters have been the subject of numerous popular and
scientific articles which cannot all be cited here. A summary of the
problems and management recommendations are found in De Meulenaer and
Raymakers (1996) and The Sturgeon Quarterly published in New
York gives recent information. Caspian populations are Endangered
(high risk of extinction in the near future - Acipenser
gueldenstaedtii, A. nudiventris, Huso huso) or
Vulnerable (high risk of extinction in the medium term future - A.
stellatus, A. persicus) (De Meulenaer and Raymakers, 1996).
In 1997, the Secretariat of the Convention on International Trade in
Endangered Species of Wild Fauna and Flora (CITES) recommended a
proposal to list all sturgeons as a species requiring protection
because of overfishing and pollution. This would result in the close
regulation of the caviar trade and perhaps a trade ban on beluga caviar. Sales of caviar in airport duty-free
shops could end as passengers in a hurry would not be able to obtain
the necessary CITES export permits or certificates from national
authorities. After 1 April 1998 shipped caviar requires export permits
or re-export certificates (Traffic North America, 1(3):14, 1998). In
the year 2000, western countries through CITES (Convention on
International Trade in Endangered Species) gave Iran, Russia, Kazakhstan,
Azerbaijan and Turkmenistan until
31 December to impose quotas on their exports in an effort to save the
sturgeon stocks. Failure to comply would result in a ban on caviar
sales in the west in the year 2002 (IRNA, 25 June 2001). Australia had already banned caviar while the U.K.
banned the import of caviar over 250 g without a permit (IRNA,
26 July 2000; The Times, 1 August 2000). Fishing for sturgeon was halted
after the spring 2001 season in all Caspian states except Iran which has a
well-managed fishery. Fishing quotas will be established after a survey in the
summer of 2001 so as to avoid a complete ban on exports (Ottawa Citizen,
19 June 2001, 22 June 2001).
By 2004, as Profitt (2004) points out, the agreement had not been fully
implemented. Pourkazemi (2006) considers most sturgeon species in the Caspian
Sea will be extinct in the near future.
Stone (2002), Stone and Mervis (2002) and Pearce (2003) give details of a
dispute between scientists and CITES which arose when fishing for beluga was
allowed in 2002. CITES endorsed Russian figures that showed beluga numbers
increased from 7.6 million in 1998, to 9.3 million in 2001 and to 11.6 million
in 2002. Scientific critics felt that there may well be less than half a million
beluga, the differences being based on estimates on how many fish escape
experimental trawling in relation to fish actually caught. The United States
banned beluga caviar imports on 30 September 2005 and Russia advocated a
moratorium on fishing of the major species (Pala, 2005). In April 2006 a global
suspension of trade in caviar and sturgeon products by CITES from the Caspian
Sea was extended indefinitely, with only one species allowed, the Persian
sturgeon from Iran, Iran being the only country that submitted harvest data for
assessment of a sustainable fishery (New York Times (www.nytimes.com),
12 April 2006, downloaded 13 April 2006). The export quota for Iran was set at 100,000 pounds of caviar.
Bemis and Findeis (1994) recommend gourmets restrict their purchases of caviar to that from
fish farms in order to preserve wild stocks of sturgeons.
There was a two-thirds to three-quarters decline in sturgeon
numbers in the Caspian from 1990 to 1995, a result of overfishing and
poaching. References cited above, The Sturgeon Quarterly (5(1/2):15, 1997) and various
newspaper and popular articles reports (e.g. Boston Globe, 8 June 1997 at www.nd.edu/~astrouni/zhiwriter/97/97060808.htm
and New York Times, 23 December 1995 at www.nd.edu/~astrouni/zhiwriter/spool/95122301.htm;
Tidwell (2001a)) give details about poaching in former U.S.S.R. waters of the Caspian
Sea. In 1996, caviar should have sold for £470/kg in Germany but was
available for £100/kg illegally (Nuttall, 1996). Caviar imports to
the U.S.A. increased by 100% from 1991 to 1996 (DeSalle and Birstein,
1996). The international market demand for caviar was 450 t in 1995
but the legal production from the Caspian Sea was only 228 t; the
deficit being made up in part by poaching (Birstein, 1996). Russia officially
exported $25 million worth of caviar in 1999 but smuggling of poached caviar was
valued at $250 million (Speer et al., 2000). As a result, natural
reproduction in the Volga River, the principal spawning ground in the
Caspian Sea has been completely destroyed (Birstein, 1996). Bickham
(1996) states that it is highly likely that the native sturgeon stocks
of the Kura River are extinct or nearly so and Khodorevskaya et al.
(1997) simply record that sturgeons no longer use the Kura and Terek
rivers. Water pollution was given as the cause for a fall in catch in Iran from
34 tons in 2000 to 9.2 tons in 2004 (Iran Daily, 27 August 2005). Legally traded
caviar fell by almost 70% between 1998 and 2003 but illegal sales probably
offset this decline (www.canada.com, downloaded 16 December 2005). The export of Iranian sturgeon was expected to
drop 20-25% in the year ending in March 2006 (Iran Daily, 25 December 2005).
However caviar exports in the 2005-2006 year were given as 18 tons in a later
report, still a drastic fall (Iran Daily, 1 May 2006). The caviar export quota
for Iran in 2006 stood at 44.3 tons (Iran Daily, 11 September 2006).
Azerbaijan increased the allowable catch from 4 tonnes to 30 tonnes
after independence and generally illegal catches made up 90% of all
sturgeon caught (Anonymous, 1996a). The yearly allowable catch for
Iranian sturgeon in 1996 was 1500 tonnes but the total catch for the
Caspian Sea probably exceeds 40,000 tonnes when all countries are
taken into account (Emadi, 1996b). Reduction in stocks was noted in
assessments carried out in Iranian waters from 1988 onward and the it
was decided to reduce the annual catch in 1996 (Iranian Fisheries
Research and Training Organization Newsletter, 14:3, 1996). Iran was
auhorised to take 90 tonnes of caviar for export in 2000 but the government
reduced this to 70 t as a conservation measure (Speer et al., 2000). A
restocking programme in Iranian waters cost about U.S.$33 million and
a buyout of 4000 fixed gillnetters cost U.S.$10 million (Bartley and
Rana, 1998b). Gill nets were trapping young sturgeon, Salmo caspius,
Barbus sensu lato spp., Rutilus spp., and Abramis brama.
Sturgeon fingerling production was 9,124,000 in 1995 and 22 million in
1996-1997 according to the above authors, 25 million according to IRNA
(2 February 1999), and 12 million according to Abdolhay and Tahori (1999). However pollution causes losses of 40-50 million
fingerlings from a production of 108 million, figures at variance with
the preceding (Tehran Times, 5 September 1999). The Iranian Fisheries
Company produced 88.1% A. persicus in 1996, 5.4% A. gueldenstaedtii,
2.7% Huso huso, 2.5% A.stellatus and 1.3% A. nudiventris (Abdolhay
and Tahori, 1999). Keyvanfar and Khanipour (1999) advocate use of trammel nets
to catch broodstock for aquaculture as fish are less stressed. TACIS (2002) and
Raymakers (2002)
give the following table for sturgeon fingerling releases in Iran (in
millions):-
| Species/Year |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
| A. persicus |
4.06 |
5.92 |
2.93 |
3.57 |
4.66 |
8.05 |
11.02 |
18.75 |
22.59 |
17.30 |
| A. gueldenstaedtii |
- |
0.04 |
- |
- |
0.30 |
0.52 |
0.67 |
0.92 |
0.42 |
0.96 |
| A. stellatus |
0.36 |
0.47 |
0.07 |
0.30 |
0.46 |
0.27 |
0.22 |
0.29 |
0.18 |
0.13 |
| H. huso |
0.14 |
0.17 |
0.45 |
0.30 |
0.49 |
0.29 |
0.34 |
1.44 |
0.69 |
0.41 |
| Total |
4.56 |
6.60 |
3.45 |
4.17 |
5.91 |
9.13 |
12.35 |
21.63 |
24.56 |
19.10 |
Salehi (2011a) gives a recent summary of fingerling production (and also costs
of production):-
| Species/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
Yearly Average |
% |
| A. persicus |
13,711,199 |
16,278,595 |
12,331,354 |
18,420,205 |
17,398,000 |
15,177,803 |
78.7 |
| A. gueldenstaedtii |
1,327,480 |
447,855 |
1,564,273 |
- |
610,000 |
1,197,307 |
4.2 |
| A.
nudiventris |
1,113,826 |
1,782,914 |
1,178,582 |
1,414,247 |
1,300,000 |
1,532,668 |
7.5 |
| A. stellatus |
226,373 |
820,136 |
1,182,902 |
196,082 |
322,000 |
635,893 |
3 |
| H. huso |
1,900,919 |
640,963 |
2,372,794 |
42,075 |
1,570,000 |
1,246,938 |
6.6 |
Abdolhay and Tahori (2006) give descriptions of hatcheries in Iran and the
process of fingerling production, including transportation and incubation
techniques, pond and tank culture, release strategies, and strategic development
plans. Trial production of larvae first occurred in 1922, reaching about 2
million in 1928 but hatchery production first began in 1971. Sturgeon fingerling
production was low between 1981 and 1986 as the focus shifted to Chinese carps
and Rutilus frisii. Brood stock are captured in rivers by beach seines or
selected from fishery stations in February-March. The fish are checked by
sampling eggs and examining germinal vesicle development. Only suitable
fish are injected with ovulation-inducing hormones in March-May over 3-5 days.
The fish are killed and the collected eggs are fertilised with diluted sperm
(1:200 with hatchery water) to avoid polyspermy as eggs have many micropyles.
Eggs are incubated in jars or troughs for 5-10 days and newly emerged larvae are
held in circular tanks. Fry are raised in fertilised ponds for 40-60 days until
they reach 3-5 g. Fingerlings are released in river deltas in June-July. Release
strategies are spot planting of all fish at once, scatter planting at several
sites in the same region and trickle planting over a period of time. Fish are
captured as adults 10-20 years later at a return rate of 1-3%.
Fingerling production in 1000s was:-
|
Year/Species |
H. huso |
A.
nudiventris |
A.
gueldenstaedtii |
A.
persicus |
A.
stellatus |
Total |
| 1993 |
301 |
no data |
no data |
3570 |
300 |
4171 |
| 1994 |
491 |
no data |
300 |
4662 |
456 |
5910 |
| 1995 |
286 |
no data |
522 |
8049 |
268 |
9125 |
| 1996 |
344 |
102 |
673 |
11,018 |
316 |
12,455 |
| 1997 |
1437 |
230 |
919 |
18,751 |
288 |
21,627 |
| 1998 |
687 |
678 |
418 |
22,586 |
181 |
24,552 |
| 1999 |
406 |
304 |
722 |
17,300 |
132 |
18,864 |
| 2000 |
1901 |
114 |
1327 |
13,711 |
226 |
17,279 |
| 2001 |
641 |
1782 |
447 |
16,278 |
820 |
19,970 |
| 2002 |
2404 |
1819 |
1816 |
12,301 |
1300 |
19,642 |
| 2003 |
42 |
1414 |
0 |
18,388 |
196 |
20,041 |
| 2004 |
1464 |
1311 |
617 |
17,412 |
314 |
21,121 |
| Total |
11,175 |
7757 |
7805 |
191,682 |
9774 |
258,567 |
Iranian sturgeons and their caviar increased in importance in the
1990s as the Russian caviar trade was taken over by a black market
system with poor attention to quality. However caviar production in
Iran fell in the 1990s through poaching and oil pollution in other
parts of the Caspian Sea. Production was 130 tonnes per year, down
from 160 tonnes up to 1989 (IRNA, 31 August 1998; Tehran Times,
13 December 1998). Caviar comprises 50% of the seafood exports from
Iran (IRNA, 21 October 1998) and formed 1.2% of Iran's total
exports for the first four months of the Iranian year in 1998 (in 1994 it was
62% (Salehi, 1999)). On the
23 October 1998, the Islamic Republic News Agency (IRNA)
reported that Iran had stopped exporting caviar to protect the
resource, this despite the number of sturgeons in the sea having risen
from 6 to 22 million over the past couple of years. The same article
reports 22 million sturgeon fingerlings stocked in the Caspian Sea by
Iran. The export of 111 tons of caviar in 1998-1999 was worth $29.5
million; catches had been reduced to save the species from extinction
(Tehran Times, 1999). The export amount was over 80 tonnes
since the beginning of the Iranian year (21 March 1999), a 30% drop in
production over the previous year (IRNA, 26 January 2000). The
1999 total export was 90 tonnes of caviar worth 70 million
deutschmarks, a monetary increase of 42% (IRNA, 4 May 2000). The 2000
export of caviar was 70 tonnes (or 71.5 t, or 80-90 t, reports vary) worth 100
million deutschmarks (or $34.4 million) with 80% going to Europe, 10% to Japan
and the rest to various other countries; in addition 100-200 tonnes of sturgeon
meat worth $2-3 million is exported annually (IRNA, 14 July 2001, 7
August 2001, 30 September 2001). The sturgeon catch was 75 t in 2002 with 50 t
being exported for U.S$30 million (IRNA, 11 June 2003). Golestan Province
produced 43% of Iranian caviar, a 17.5% increase presumably in 2000 over the
1999 catch. There are 295 fishermen using 91 fishing boats (IFRO Newsletter, 29:4, 2001).
Sturgeon stocks were evaluated in Iranian waters in 2000 (M. Moghime and F.
Parafkandeh Haghighi, 5th International Symposium on Sturgeon, Iranian Fisheries Research
Organization, 9-13 May 2005, Ramsar; Haghighi, 2006; Moghime, 2006). The catch was 855 t yielding 92.5 t of
caviar, with Acipenser persicus comprising 472 t, A. stellatus 201
t, H. huso 105 t, A. gueldenstaedtii 48 t and A. nudiventris
31.8 t. The catch-per-unit-effort was A. gueldenstaedtii (0.285 kg),
A. persicus (2.296 kg), A. nudiventris (0.089 kg), and A.
stellatus (2.941 kg). Mature females comprised A. gueldenstaedtii
(80.0%), A. persicus (71.8%), A. nudiventris (51.3%), A.
stellatus (74.7%) and H. huso (67.4%). The gonadosomatic value in
terms of body weight was A. gueldenstaedtii (9%), A. persicus
(11%), A. nudiventris (8%), A. stellatus (14%) and H. huso
(2.8%). The catch in Gilan and Golestan provinces was A. stellatus (11%)
and H. huso (35%) of the total catch. In Gilan, the catch was made up of
A. gueldenstaedtii (44.3%), A. persicus (16.4%) and A.
nudiventris (3.8%) and in Golestan these values were 16%, 72% and 0.9%
respectively. The average age in Gilan and Golestan respectively was A.
gueldenstaedtii (15.5 and 18.5 years), A. persicus (20 and 19.2
years), A. nudiventris (15 and 19.3 years), A. stellatus (13.8 and
13.2 years and H. huso (15.8 and 18 years).
Tavakoli et al. (2007)and Kor et al. (2008) surveyed stocks in
the southern (2004-2005) and northern (2006) Caspian Sea respectively. In
the southern Caspian Sea catch of 288 fish total, catch per unit effort was 2
fish in summer and 1.38 fish in winter. The most abundant species was
Acipenser persicus with 1.67 fish per trawl in summer (142 fish) and 0.88 in
winter (75 fish). A. stellatus was 0.22 and 0.48 fish. No Huso huso
were caught in winter and only 4 fish in summer. A. nudiventris comprised
only 4 fish too and and A. gueldenstaedtii 3 fish, both in total. Kor
et al. (2007) examined the population structure of sturgeons in the coastal
waters of Mazandaran, less than 10 m deep, for 2003-2005. The number of fish
captured in 2003-2004 was 301 with catch per unit effort (CPUE) being A.
persicus 4.07, A. stellatus 0.58, A. nudiventris 0.22, and
A. gueldenstaedtii 0.15, and in 2004-2005 the catch was 412 fish with CPUE
A. persicus 6.15, A. stellatus 0.23, A. nudiventris 0.12,
A. gueldenstaedtii 0.35, and Huso huso 0.02.
The world's leading importer of caviar, Caviar House, with an
annual turnover of $100 million took 85% of its caviar from Iran
(Lindberg, 1994; Pala, 1994). The value of the caviar fishery in Iran
was estimated at U.S.$45 million (Bartley and Rana, 1998a; 1998b) and
is the main fish product exported with an international cultural and
culinary significance. The caviar industry in Iran is a state monopoly
under strict control and has not suffered from poaching to the same
extent as happened in the former U.S.S.R. after the collapse of
central authorities. There has been some smuggling reported via Bandar
Abbas to Ras al Khaimah across the Gulf and re-labelling of
Azerbaijani caviar as Iranian to fill Iranian contracts with the U.A.E.
An illegal trade in "bazaar" caviar reached a peak of 70
tonnes in 1983, about 50% of the legal exports (Taylor, 1997). This
caviar was processed poorly in primitive tins with sealant rings made
from old tyres; consequently the price for this product was low. The
Iranian government actively sought to suppress this trade and after 10
years of effort reduced smuggling to 2-4 t annually, a level similar
to that prior to 1979. In 2003 however, 3.8 t of smuggled sturgeon fish and
caviar were reported as confiscated for the previous year (ending 20 March) in
Mazandaran (IRNA, 21 April 2003). Evidence of Iranian control of the industry is
seen in the 1994 setting of a minimum catch size limit of 1 m on all
sturgeon species and limiting fishing sites along the Caspian coast to
90 (Josupeit, 1994; De Meulenaer and Raymakers, 1996). Additionally
Iran now stocks more sturgeons from farms than it catches (The
Times, London, 8 July 1998). However the BBC News (6 May 1998)
reports declines in catches of sturgeon over the past 5-10 years.
The illegal market in caviar has been estimated at £500 million with some caviar fetching up to
£20,000 a kilogramme (The Times, 28 December 2006). In Britain, caviar tins must indicate
their exact source and without this label will be seized by Customs. The label
will carry a species code, source of the caviar, country code, year of harvest,
processing plant registration number and lot identification number, all in an
attempt to regulate and eliminate sales of smuggled caviar. Much of the smuggled
caviar is sold under the counter or to those who have pre-ordered it, or by
shops that then state they were unaware of its illegal status.
Although caviar is the main market item for sturgeons, Iran is
investigating the use of fillets and smoked and salted A. stellatus
in vacuum packs for export (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 45-46, 1997).
Smoked, marinated and canned sturgeon, smoked sturgeon in vegetable oil and
frozen fillets are now available (2001) from several Iranian companies.
Javanmard and Taghavi (2002) investigated the microbiological and chemical
characteristics of these products and only one had a total coliform count more
than the European Community standard. Gelatin has also been produced from sturgeon
fish skin on an experimental basis
(Iranian Fisheries Research Organiztion Newsletter, 30-31:6, 2002;
Koochakian Sabour et al., 2001).
All sturgeon species in the Caspian Sea basin are listed as
"endangered" or "vulnerable" and are maintained in
part by hatchery stocks (http://www.sturgeons.com/htdocs/status.html).
Survival and growth of sturgeon fry in the Caspian Sea is reviewed in
Farsi by Aslaanparviz (1992).
The countries of the Caspian littoral are attempting to conserve
their sturgeon stocks. Even the Swiss company Caviar House has established a
hatchery in Iran to increase stocks (Anonymous, 2001a). An agreement for the "Preservation and
Exploitation of Live Resources in the Caspian Sea" was made
between Iran, Russia, Azerbaijan, Turkmenistan and Kazakhstan in 1996.
Luk'yanernko et al. (1999) point out the need for the agreement to
recognise that sturgeons are sustained by an ecosytem involving the whole
Caspian Sea and the inflowing rivers, that there must be an absolute ban on
uncontrolled fishing for sturgeon in the sea and that national quotas must
reflect the real contribution of a a particular state to overall sturgeon
stocks. Without adequate measures, these authors predict extermination within
5-7 years. Export of caviar is made a monopoly of the governments concerned in an
effort to minimize smuggling of low quality caviar. Jenkins (2001) gives reasons
why an international trade ban would not necessarily help conserve the sturgeons
- most poached caviar is sold within Russia, for example. Sturgeon catches
are restricted to rivers and their estuaries and open-sea trawling is
banned. The five countries are investing $150 million in a fish farm programme to save the sturgeon from extinction: Russia will have 10
new farms and renovate 8 farms on the Volga River, and both Kazakhstan
and Iran have a new farm (Abzeeyan, Tehran, 7(4):II-III, 1996; The
Sturgeon Quarterly, New York, 4(4):1, 1996; newspaper reports). Russian
strategies for conservation of sturgeon are reviewed in Artyukhin et al.
(1999) and the status of the Russian sturgeons is given in Vaisman and Raymakers
(2001). Some sturgeon species are now on Appendix 2 of the U.N. Convention on
International Trade in Endangered Species of Flora and Fauna (CITES)
in an effort to control the import and export of meat and caviar
(Pearce, 1997). The U.S. Fish and Wildlife Service, in an attempt
to combat overfishing of sturgeons, now requires valid CITES permits
for imported caviar (Anonymous, 1998a). DNA tests will be used to
confirm the species of sturgeon listed on the shipment and to
eliminate illegal mixtures with inferior quality roe. Even cats are now used to
detect smuggled sturgeon in Russia. A cat named Rusik is able to detect sturgeon
hidden in trucks better than sniffer dogs (National Post, 9 July 2003, p. A12).
The number of adult fish in the Caspian Sea had declined from 142
million in 1978 to 43.5 million fish in 1994. Ivanov et al. (1999) and
Khodorevskaya and Krasikov (1999) review the status of stocks in the Caspian.
Marked declines are evident and only the Iranian catches are reasonably stable
from 1977 to 1994. All species studied in
Iranian waters had a very low percentage of fish older than 20 years,
are evidently in need of protection (Iranian Fisheries Research and
Training Organization Newsletter, 16:4-5, 1997). An initiative to
make the sale of caviar from threatened sturgeon species illegal is
being proposed by the Species Survival Commission and the IUCN (The
Sturgeon Quarterly, New York, 4(4):1, 1996; Morris, 1997). Part of
this initiative would involve genetic testing of the caviar as a means
of identifying the species of sturgeon. Paddlefish eggs from Montana,
U.S.A. costing less than $5 an ounce have been repackaged as beluga
caviar in Russia and eastern Europe and sold in the U.S.A. for $50 an
ounce. The U.S. Fish and Wildlife Service was to begin monitoring the
caviar trade on 1 April 1998 using DNA tests (U.S.A. Today, 18
November 1997, internet edition). Birstein et al. (1998)
describe a molecular technique for identifying the species source of
commercial caviar (see also Brainard (1998)). They found 23% of
species designations by caviar suppliers to be incorrect, indicating
possible illegal harvesting and poaching. The Iranian Fisheries
Research and Training Organization Newsletter (20:4, 1998) also
reports on nuclear DNA amplification and a marker which distinguishes
species. Additional research is being carried out on egg
identification using ultrastructural characteristics (L. Debus and M.
Winkler, 1998, www.uni-rostock.de).
Sturgeons have been fished since the Neolithic, perhaps 6000 or
more years ago (Tsepkin, 1986) but only in recent years have the
stocks declined significantly. Historical records show it was possible
to catch 500 Huso huso weighing 600-1000 kg in about 2 hours in
the Volga delta at the end of the eighteenth century (Birstein, 1993).
All the sturgeon species were bigger on average and lived longer than
now based on archaeological excavations (Tsepkin and Sokolov, 1971).
The sturgeon catch in the Caspian Sea declined from 27,400 tonnes in
1977 to 8,900 tonnes in 1990 (Vlasenko, 1995). The catch in 1993 was
only 4,200 tonnes because of poaching and pollution of the Volga River
(The Sturgeon Quarterly, 3(1):12, 1995). An estimated 90% of
the Caspian sturgeons are killed before they mature (Platt, 1995).
Catches in Russian waters of the Caspian Sea declined from 7106 tonnes
in 1992 to 3426 tonnes in 1993 to 2960 tonnes in 1994 but 90% of the
real catch is unreported (16,700 tonnes were reported in 1983 for
comparison). The number of adult sturgeons in the Caspian Sea is
estimated to have declined from 142 million fish in 1978 to 43.5
million fish in 1994 (De Meulenaer and Raymakers, 1996). The Caspian Sea
Sturgeon Ranching Programme of the former Soviet Union helped to sustain
fisheries but declines still occurred (Secor et al., 2000).
Catches in Iran, however, increased over a five year period,
perhaps because of heavier fishing pressure. Sternin and Doré (1993)
cite figures for 1986-1990 of 1690 tonnes, 1759 t, 1851 t, 2051 t and
2021 t, while U.S.S.R. catches over the same period were 21,817 t,
20,991 t, 19,027 t, 16,880 t and 15,056 t. A conflicting study noted a
decline from 122,000 sturgeons caught in 1986 to 68,000 in 1993 (Abzeeyan,
Tehran, 6(5, 6):IV-V, 1995). De Meulenaer and Raymakers (1996)
summarise Iranian catches as 700 to 2500 t in the twentieth century,
peaking towards the end of the 1960s, falling to 1000-1500 t in the
1970s and increasing from 1450 t in 1982 to a 1991 high of 3036 t but
falling off rapidly to 1700 t in 1994. Josupeit (1994) gives catches
in Iran in tonnes from 1982 to 1992 as 1450, 1288, 1557, 1650, 1690,
1759, 1851, 2051, 2645, 3036 and 2692 t. The commercial sturgeon catch
in the Safid River delta fell from 6700 tons in 1961 to less than half
a ton in 1993 (http://www.oneworld.org/patp/pap_overview.html).
Spawning may no longer take place in the Safid River (De Meulenaer and
Raymakers, 1996). Zanusi (1995) maintains that over 40% of the total
sturgeon fishing in the Caspian Sea is centred on Bandar-e Torkeman in
Mazandaran, presumably including the acknowledged black market in
sturgeon products. Lewis (1980) gives some information about the
Iranian black market in caviar shortly after the Islamic Revolution
before controls were re-established. A 400 g tin was selling in Paris
black market for $40 compared to $310-315 for the best Russian beluga. Caviar
production in the three Caspian coast provinces of Iran for the 1990s were as
follows in kg after Nezami et al. (2000):-
| Year/Province |
Gilan |
Mazandaran |
Golestan |
| 1991 |
75,974 |
78,713 |
128,446 |
| 1992 |
81,520 |
80,758 |
99,336 |
| 1993 |
51,480 |
58,543 |
83,026 |
| 1994 |
40,368 |
52,162 |
87,576 |
| 1995 |
37,241 |
43,831 |
70,154 |
| 1996 |
41,743 |
41,432 |
79,063 |
| 1997 |
28,641 |
42,329 |
58,304 |
The problem of overexploitation of sturgeons is compounded by their
long life span and their use of rivers as spawning grounds such that
they are easily caught on this migration from the sea. The migration
and spawning is timed differently between species and populations
within species. Some sturgeons migrate long distances up rivers while
others have a shorter migration. Eggs are deposited on stony or gravel
bottoms and hatch after a short incubation. As an example, a study of
sturgeon migrations in the Gorgan and Tajan rivers of Iran showed a
movement of 2 out of 28 fish caught at one station reached the second
station in the Gorgan and no tagged fish reached higher stations in
the Tajan - the rest were caught by fishermen (Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization,
Tehran, p. 53, 1997).
Ramin (1998) studied migration in the Safid River over 35 days in April-May,
from the mouth to 30 km upriver for A. gueldenstaedtii, A. persicus
and A. stellatus. Shallow water caused by sand-clay deposits and illegal
fishing did not prevent successful migration. It was recommended that the Manjil
Dam be used to regulate water flow and a total ban on fishing, especially at the
mouth, during the March-May spawning season be implemented.
In 1998 the comb jelly, Mnemiopsis leidyi, reached the
Caspian Sea via ship ballast and newspapers speculated that the
sturgeon populations would be affected, although how was not
specified.
The young migrate downstream to feed and grow in the sea. Old
reports have sturgeons overwintering in deeper parts of rivers, in a
kind of torpor and with a viscous substance coating the body (Baird,
1873). The barbels are highly sensitive and, as soon as they detect
food, the tubular mouth protrudes to suck in the prey. Food is benthic
organisms although some are predators on larger fishes. Young sturgeon in Iran
feed predominately on polychaetes while crustaceans are a minor food item,
probably caused by lower oxygen conditions favouring the former (Haddadi
Moghaddam and Negaresten, 2003). Pourgholam
(1994) reports the coelenterate Polypodium hydriforme from
sturgeons caught on the Babol Sar and Bandar-e Torkeman fishing
grounds in Mazandaran where up to 25.6% of fishes are infected,
particularly Huso huso and Acipenser gueldenstaedtii.
This parasite destroys the eggs of sturgeons, affecting reproductive
success and the caviar industry (see also Raikova (2002)). Incubated eggs of sturgeons are
susceptible to various species of fungi, with up to 70-90% of eggs
being lost (Czeczuga et al., 1995). Czeczuga et al.
(1995) report 43 species of fungi on eggs of sturgeons from Russian
and Iranian Caspian Sea samples immersed in water from a Polish river,
lake and pond. Huso huso and Acipenser gueldenstaedtii
persicus (sic) eggs carried the fewest species of fungi,
about half the load of other sturgeon species. Ghoroghi (1996) reports
metacercariae of Diplostomum spathaceum in the lens of 22% of
fingerlings on the Shahid Beheshti Fish Farm causing weight loss and
mortality. External parasites on sturgeons include Pseudotracheliastes
stellatus, Nitzschia sturionis, Diclybothrium armatum,
Cystoopsis acipenseris and Diplostomum spathecum with the highest
prevalence in Huso huso at 60% and the lowest in Acipenser persicus
at 13.9% (A. Hajumoradloo in 5th International Symposium on Sturgeon, Iranian
Fisheries Research Organization, 9-13 May 2005, Ramsar). Ghaemi et al.
(2006) found strains of mycobacteria in Iranian sturgeons and Mycobacterium
marinum can cause fish tank granuloma, a disease in humans although none was found in fishermen.
Many sturgeons in former Soviet waters of the Caspian Sea have
developed fatal diseases associated with chemicals such as phenols,
waste fluids and air from gas production facilities associated with
the petrochemical industry. Both the sturgeon and their caviar are now
inedible. Iranian sturgeons are believed to be less affected but since
sturgeons migrate they are susceptible to extra-territorial pollution (Golub, 1992).
Sturgeons are some of the most important commercial species in the
world, with 90% of the total catch coming from the former U.S.S.R. and
only 6% from Iran (but see later under Acipenser gueldenstaedtii
where Iranian production of caviar increased in the 1990s). Over 90%
of all sturgeons are caught in the Caspian Sea. The proportion of
catch is heavily weighted towards the former U.S.S.R. (compared with
Iran in parentheses) with figures from 1971 to 1988 as given by
Sternin and Doré (1993) being 19,100 tonnes (2400 t) for 1971, 20,400
t (2200 t) for 1972, 24,958 t (1801 t) for 1978, 26,322 t (1578 t) for
1979, 26,697 t (1429 t) for 1980, 26,452 t (1496 t) for 1981, 25,704 t
(1450 t) for 1982, 25,570 t (1500 t) for 1983 and 18,470 t (1700 t)
for 1988. The Volga River and its delta provided 75% of the commercial
sturgeon harvest in the Caspian Sea with Acipenser gueldenstaedtii
making up 60-70% of this amount, A. stellatus about 30% and Huso
huso 5-6% (Khodorevskaya et al., 1997). Williot and
Bourguignon (1991) summarise sturgeon catches in Iran from FAO data
for 1965 to 1987 as ranging from a low of 1429 t to a high of 3000 t. Abdolhay
and Tahori (2006) summarise catches as follows:-
| Year |
Total catch
(tonnes) |
A.
stellatus (%) |
Osetra*
(%) |
H. huso
(%) |
| 1972 |
1500 |
34.0 |
36.3 |
29.7 |
| 1991 |
3036 |
49.5 |
41.0 |
9.5 |
| 1994 |
1700 |
49.5 |
41.0 |
9.5 |
| 1997 |
1300 |
35.8 |
54.3 |
9.9 |
| 2000 |
1000 |
35.8 |
61.0 |
3.5 |
| 2001 |
870 |
28.2 |
69.3 |
2.5 |
| 2004 |
600 |
10.7 |
74.6 |
14.7 |
* presumably includes A. persicus and A. gueldenstaedtii
Only 5% of Iranian caviar is consumed in that country, the rest
being exported. Domestic prices are very high at about U.S.$340 per kilogramme
and the caviar is rationed to 100 g per person. Contraband caviar can be bought
at about half this price around Bandar Anzali and at least 30 t are smuggled out
of the country each year (The Daily Star, 7 December 2004, www.dailystar.com, downloaded 17
December 2004). In 1996, 95 t out of an estimated 120 t catch was
exported although formerly as little as 38.7% was exported as in 1978.
Iran is the chief exporter to the European Union, the weight varying
from 95 to 125 t from 1988 to 1994 (De Meulenaer and Raymakers, 1996).
Caviar exports by year for Iran are given by these authors as:-
|
Year |
1988 |
1989 |
1990 |
1991 |
1992 |
|
tonnes |
225 |
249 |
226 |
225 |
169 |
|
U.S.$1000 |
42,155 |
47,865 |
46,005 |
53,800 |
42,004 |
|
U.S.$/kg |
187 |
192 |
204 |
239 |
249 |
The export volume of caviar for 1997-1998 was 105 tonnes worth 62
million German marks (= U.S.$34 million) (IRNA, 16 March 1998).
Prices outside Iran have fluctuated widely because of large amounts
of illegal and often poor quality caviar flooding the world market.
Caviar exports are declining in the 1990s reflecting, it is believed,
the loss of sturgeon stocks in the Caspian Sea (De Meulenaer and Raymakers, 1996).
Caviar is the main product but the flesh is also eaten (a religious
ruling was made in the 1980s to the effect that Iranian ichthyologists
had determined sturgeons to be fish with scales - see below). Early
reports of poisoning from sturgeon eggs have been attributed to poor
preservation and consequent bacterial contamination (Halstead,
1967-1970). The milt of Acipenser sturio contains a toxic
substance known as "sturin" and although this species does
not occur in Iran a similar toxin may occur in Iranian Acipenser (Coad, 1979b).
The swimbladders of sturgeons have been converted to isinglass, a
transparent gelatin used in a variety of products including as a wine
and beer clarifier, in jams and jellies and in glass and pottery. Gmelin (2007)
mentions that in 1770-1774 people along the Langerud were catching large numbers
of sturgeons for their isinglass only, the caviar and flesh not been used. Sabour (2006)
found the swimbladder in Iranian sturgeons to weigh 250-285 g in H. huso,
35-92 g in A. stellatus and 85-160 g in Acipenser spp and could be
was processed to isinglass at 15-20%. Koochekian et al. (2006) found a
higher percentage production of isinglass from A. persicus/A.
gueldenstaedtii than in Huso huso or A. stellatus. Recently Iranian
scientists have investigated production of leather from sturgeon skin
(Iranian Fisheries Research and Training Organization Newsletter,
4:2, 1994; Davarzani, 1995; Iranian Fisheries Research Organization
Newsletter, 22:2, 2000; Iran Daily, 17 January 2006). An estimated 1 million square feet of
leather could be produced and used in handicrafts, book binding,
waterproof products and ornaments.
Various methods to enhance the sturgeon fisheries have been investigated in
Iran. Some are given under the Species Accounts and others are summarised here.
Experiments with pen culture in Gorgan Bay have been carried out to
increase production and with cross-breeding Huso huso and Acipenser
stellatus to create new commercial and resistant stocks (Iranian
Fisheries Research and Training Organization Annual Report,
1992-93). A Farsi review of sturgeon culture is given by Rasoli
(1992). Even surgical procedures under anaesthesia have been tried to
remove eggs through a 15-20 cm incision as part of attempts to
increase caviar production (Mokhayer, 1993; The Times, London,
8 July 1998). Ultrasonagraphy has been used successfully to
distinguish males, females and immature fish without damaging them (Vajhi,
Moghim, Veshkini and Masoudifard (1999) www.mondialvert99.com,
downloaded 31 May 2000; Moghime, 2006). The accuracy was 97.2% for A.
stellatus and 100% for A. gueldenstaedtii, A. nudiventris and
H. huso. Vacuum pumps have also been used to breed
female Acipenser nudiventris and male A. stellatus. The
fish are anaesthetized with xylazine hydrochloride and then eggs and
sperm are pumped out, the advantage being that females can be returned
alive to the sea (Iranian Fisheries Research and Training
Organization Newsletter, 13:5, 1996). Bahmani et al. (2001)
compared haematological parameters in Acipenser persicus and Huso huso
and how these changed with age. Haemtaological indices give insight into
the physiological condition and aid in the selection of broodfish.
Cryopreservation of sperm has been carried out as stripping fish late in the
season is difficult. Sperm in liquid nitrogen with an extender is viable for 1.5
to 2 years (M. Moghim and H. N. Moghadam in 5th International Symposium on
Sturgeon, Iranian Fisheries Research Organization, 9-13 May 2005, Ramsar;
Moghadam, 2006). The colour of gill nets used in the capture of sturgeons has been investigated with
blue nets having a yield of 42.6%, white 29.8% and green 27.5% (5th
International Symposium on Sturgeon, Iranian Fisheries Research Organization,
9-13 May 2005, Ramsar). Studies on the fingerling production of hatcheries
include the nature of the phytoplankton community and the benthic biomass,
parasitic infections (e.g. Diplostomum sp. on the eyes and Trichodina
sp. on the gills were noted at an incidence of 25% and 35.85, productivity
(6,509,185 fingerlings produced from 31 March and 28 July 2000 in two hatcheries
with some transfer of Huso huso fingerlings from another hatchery),
survival rates (56.7% and 25.2% for A. persicus and H. huso
respectively), and growth rate and condition factor (generally low). Kami et
al. (2005) studied the biology of pond turtles (Emys orbicularis)
which live in culture ponds along with sturgeon. One dietary item was
Acipenser persicus. The use of probiotics, microbial cells in the diet, used
to improve health and thus enhance quality of farmed fish is of potential use in
sturgeons as reviewed by Askarian et al. (2006). Bahmani (2006) used both
histology and haematology on Acipenser persicus, A. gueldenstaedtii
and Huso huso to determine physiological condition of fish in ponds
and rearing tanks, comparing the results with natural conditions (similar) and
finding that fibreglass tanks were more suitable than rearing ponds. Banadani (2006) examined
the environmental conditions in the Gorgan River, a major site for release of
sturgeon fingerlings. Mohseni (2006) studied the effect of stocking density of
eggs and larvae in incubators on their survival, growth and appearance of
deformities. Increased density reduced survival and growth and increased
deformities. Parandavar (2006) compared production of sturgeon from broodfish
maintained on farms to those produced from fish taken from the wild. Salehi
(2006) analysed the economics of sturgeon fingerling production and found labour
costs were 55%, food and fertiliser 14%, maintenance 7% and fertilised eggs 5%.
A single fingerling cost 992 rials to produce in Iran, varying between 447
and 1224 rials among hatcheries. Yousefian (2006) gives details of the
production of fingerlings at the Shaid Rajii Fish Farm in 2002. This farm
produced 2,898,086 or 93.27% of the fingerlings released into the Tajan, Larim,
Goharbaran and Sardab rivers. These fingerlings had an average weight of 3.58 g
and condition factors were 0.4 for Acipenser persicus, 0.37 for A.
gueldenstaedtii and 0.31 for A. stellatus, in total and average grade
for the condition factor. Fazlei (no date) summarised the number and quality of
fingerlings released into Mazandaran and Golestan provinces. The most important
rivers for release were the Gorgan (8,659,377 fingerlings, average weight 2.55
g), Tajan (1,453,410, 4.12 g), Larim (1,211,875, 3.4 g) and Goharbaran (743,561,
3.09 g). A. persicus comprised 87.7% of the fingerlings, A.
gueldenstaedtii 6.6%, H. huso 3.3% and A. nudiventris 2.4%.
The International Sturgeon Research Institute has developed a food formula based
on Iranian sturgeon species. Previously food for aquaculture came from Europe
and the domestic version was demonstrated to be superior
(Iranian Fisheries Research
Organization Newsletter, 58 & 59:2, 2009). The hybrid
sturgeon known as bester (female beluga x male sterlet) has been investigated
for expanding sturgeon culture in Iran. Growth was significantly better than in
beluga
(Iranian Fisheries Research
Organization Newsletter, 58 & 59:4, 2009). Jafarian et al. (2009)
studied the use of probiotic bacilli to encapsulate Artemia urmiana
nauplii and the yeast Saccharomyces cerevisiae used to encapsulate
Daphnia magna, Artemia and Daphnia being used as live food for
sturgeon larvae. Both treatments increased growth parameters and feeding
efficiency in A. persicus, A. nudiventris and H. huso.
Lake Orumiyeh has been used as a source for Artemia urmiana or brine shrimp to be used as a
live food in sturgeon aquaculture (Azari Takami, 1987; 1993). Brine shrimp were
found to be a better food than white worms or Daphnia, being cheaper and
easier to prepare, easier to store as cysts, sturgeon fry showed better growth,
pathogens were less, mortality was lower and yield higher. Since 1972 almost 50%
of fry diet has been brine shrimp. The large mouths of sturgeon fry enable them
to take brine shrimp nauplii and even adults a few days after yolk-sac
absorption. Fry are grown to 100-120 mg within 7-10 days and then released into the sea.
Anonymous (1961b) reported on the caviar industry in Iran which at
that time was about 5-6% of the world supply. The Food and Agriculture
Organization of the United Nations in their Yearbook of Fishery
Statistics reported catches of sturgeons from 1980 to 1985 as 1429,
1496, 1450, 1288, 1557 and 1650 tonnes respectively. Soviet catches of
sturgeons in all waters, not just the Caspian Sea, ranged from 22,772
to 26,697 tonnes for the same period. Petr (1987) summarised FAO
statistics for Iran and gave mean landings of sturgeons as 2300 tonnes
(1964-1970), 1800 t (1971-1975), 1500 t (1976-1980), and 1774 t
(1980-1985) but some of this data is very approximate being repeats of
a 1500 t value as an estimate (see also above for more figures). A
pamphlet from the Ministry of Jahad-e Sazandegi (= Construction
Crusade or Rural Development), which is charged with fisheries in
Iran, gave catches for "caviar fish" of 3036 tons
(presumably tonnes) in 1991 and 2692 tons in 1992. The catch in 1995
was 995 tonnes yielding 134 tonnes of caviar with 74% of the catch
from Mazandaran province (http://netiran.com:80/news/TehranTimes/html/95122503TTEC.html).
Other news reports give the 1995 catch as 142 tonnes of caviar, in
1996 112 t and an estimated 140 t in 1997. The 200 t of caviar
produced in 1992 was worth $100 million through export while the 1997
catch was worth only $60 million despite a 50% increase in price. The
Tehran Times (30 May 1998) reported that caviar production was reduced
from 220 tonnes to 40 tonnes during the previous 6 years to preserve
stocks. The allowable catch in 2003 was set at 676.4 t for Iran, a decrease from
685 t in 2002, with caviar exports set at 78.8 t. Figures for other Caspian
states were Azerbaijan 130 t (9.1 t of caviar), Russia 429 t (30.3 t),
Kazakhstan 216 t (23.18 t), and Turkmenistan 56.25 t (5.85 t)(IRNA, 28 December 2002).
Caviar from Iran commanded a higher price than that from the former
U.S.S.R. in the 1990s (Christie, 1995). Catches in the 1952-1957
period yielded an annual average yield of 120 tons (sic,
possibly tonnes here and below) of caviar (Kayhan International, 1
December 1962) which agrees closely with the figure given by Job
(1961a) of 90-115 tons (sic) annually. Catches from 1965/66 to
1968/69 in Iran rendered 208 to 219 tonnes of caviar annually from
1996 to 2290 tonnes of the three main species fished (A.
gueldenstaedtii (presumably including A. persicus), A.
stellatus and Huso huso)(Andersskog, 1970). The catch in
1961-1962 was 170 tons (or 178 tons, V. D. Vladykov, in litt.,
1966; differing data is not unusual as effectiveness of information
gathering varies) and this was the first season when exports to the
U.S.A. exceeded that to the former U.S.S.R., 56 to 46 tons, with 58
tons going to Europe and about 10 tons consumed locally. The caviar
yield in 1956-1957 was low, at 134 tons (or 137 tons, V. D. Vladykov, in
litt., 1966) and averaged 120 tons from 1952-1957 (Kayhan
International, 1 December 1962), a decline over levels prior to
dissolution of the Iran-Soviet company. White (1988) reported a caviar
export of 150 tonnes from Iran with a value of U.S.$20 million out of
a 250 tonnes annual production. Caviar yield in airtight containers
was 233 tonnes (1981), 204 t (1982), 222 t (1983), 247 t (1984), 304 t
(1985), 283 t (1986), 296 t (1987), 281 t (1988), 286 t (1989), and
290 t (1990) (Sternin and Doré, 1993). Production of caviar in the
1990's dropped steadily from 160 tonnes to 120 tonnes as the Caspian
became more polluted (Tehran Times, 5 August 1999) and the
catch for the year ending in March 2000 was expected to be less than
100 tonnes (Reuters News Service, downloaded 1 September 1999).
Pollutants from Russia, Azerbaijan and Kazakhstan include oil spillage
from old equipment at offshore sites and 12 million cubic metres of
sewage from the Volga. The sewage includes toxic PCBs, phenol, heavy
metals, dioxins and DDT as well as household, agricultural and
industrial wastes. A 10-year ban on sturgeon fishing would have to be
placed into effect to allow stocked sturgeon to mature and breed. Research
on qara burun (A. persicus) and uzun burun (A. stellatus) in Iran
has shown heavy metal (cadmium, copper, zinc, lead and mercury) density in
caviar and flesh to be 10 times less than the global safety standard (IRNA,
15 January 2002; IFRO Newsletter,
28:2, 2001). Pourang et al. (2005) examined all five sturgeon species in
Iranian waters and found all toxic trace elements (Cd, Cu, Pb and Zn) to be
markedly below international guidelines for human consumption. Kajiwara et al. (2003) demonstrated contamination by
organochlorines in Iranian sturgeons. DDT and its metabolites predominated at
180-18,000 ng/g on lipid weight followed by PCBs at 110-1900 ng/g. Generally Huso
huso was the most contaminated species and contaminant concentrations were
higher in Azerbaijan and Kazakhstan than Iran, the latter having fewer oil
wells. Gelodar (2006) evaluated four caviar processing plants for their
hygienic standards using the Hazard Analysis and Critical
Control Point (HACCP), an internationally recognized food safety system. Those plants following
the European Community code had decreased their contamination levels.
70% of Iranian caviar is produced in Mazandaran, 130 tonnes in 1994
(Abzeeyan, Tehran, 6(5, 6):III, 1995) although this conflicts
with a report from IRNA for 2 May 1998 where Mazandaran has 35%
of the total Iranian output at 44 tonnes for 1997-1998. 95% of the
Mazandaran caviar is exported along with 60 of 260 tonnes of sturgeon
meat (IRNA, 2 May 1998). The Bandar-e Torkman fisheries
organization in Golestan Province (eastern Caspian Sea) planned to
process 360 tonnes of sturgeon and 48 tonnes of caviar in 1999-2000 (IRNA,
14 December 1999). Newspaper reports in 1995 gave a value of U.S.$40
million for caviar exports from Iran; another report gave U.S.$50
million for 250 t (Food and Agriculture Organization, Fisheries
Department, 1996). This is less than the value of half a day's oil
sales but the caviar fishery is a national symbol (Christie, 1995). Mazandaran
produced 17 tons of caviar in 10 months in 2003-2004 as well as 140 tons of meat
(www.iranmania.com, downloaded 4 October 2004). In
the Iranian fiscal year ending 20 March 1998 Iran exported 105 tonnes
of caviar worth about U.S.$11 million (Anonymous, 1998b). The 2003 allowed share
for Iran was 78.8 t from a catch for the whole Caspian Sea of 148 t (IRNA, 22 September 2003).
The quota for all Caspian caviar in 2004 was 125 tons (www.nytimes.com,
downloaded 12 October 2004).
The sturgeons were little used after eggs were extracted for caviar
although they were sometimes served in small restaurants along the
Caspian coast (personal observations; remarkably tough and tasteless
too!). Sturgeons were "haram" in Iran, forbidden for
religious reasons as scaleless fish although this has been reversed
(Caddy, 1984; Anonymous, 1989; saffron, 2002). Most flesh was exported to Russia (RaLonde
and Walczak, 1970b) although some is dried and pickled for local
consumption (De Meulenaer and Raymakers, 1996) or eaten freshly
grilled (personal observations). Fraser (1834) noted thousands of
sturgeon carcasses lying on the Safid River banks, discarded after
removal of eggs for caviar and swimbladders for isinglass. Export
prices in 1995 ranged from $5.00 per kg of fresh sevryuga fillet to
$14.50 per kg for smoked beluga (fil mahi) fillet (Abzeeyan,
Tehran, 5(9):V, 1995). Shilat now markets sturgeon head-on or
headless, gutted, frozen or in any form required by customers. The
average processed weight is about 20 kg for Huso huso, 8 kg for
Acipenser gueldenstaedtii (probably includes persicus)
and 6 kg for Acipenser stellatus. The meat is served roasted or
smoked (Shilat advertisement in Seafood International, December 1995).
Research has been carried out in Iran on products derived from left-over parts
of sturgeons, the intestines for fish sauce, and skin for gelatin (Sabour et al., 2006).
Capture methods, in the early twentieth century, involved large iron-barbed
hooks attached to ropes stretched across the river mouth to foul-hook the
sturgeon or, further upstream, poles 6-8 feet long armed with an iron hook used
to gaff the sturgeon (Fortescue, 1920). Sturgeons may be caught more recently by large shore seines but mostly they are
taken by gill nets set 1-3 km out to sea although De Meulenaer and
Raymakers (1996) refer to 300 m fixed nets in rivers with passage
space at the sides and bottom as the only authorised method in Iran.
Trawling in the sea is not allowed in Iranian waters. Sturgeons are
taken from March to June and from September to November in each year
(Christie, 1995). The autumn season is best for A. gueldenstaedtii
(and presumably A. persicus) while the spring season is best
for Huso huso and A. stellatus (De Meulenaer and Raymakers, 1996). Autumn is the main season when the sturgeons migrate
to the southern Caspian Sea (De Meulenaer and Raymakers, 1996). The
draft "Agreement on the Conservation and Utilization of the
Biological Resources of the Caspian Sea" in 1995 prohibited
sturgeon fishing in the open Caspian Sea except for traditional
methods by Iran near its coast within quota limits (Vinogradov in Glantz and Zonn, 1997).
Gill nets used to capture bony fishes, mainly cyprinids, are
responsible for an increase in malformations observed in sturgeons in
recent years (Mehdizadeh, 1993). Fins are broken or cut, rostrums
(snouts) deformed and net fragments embedded in flesh. Gill nets were
prohibited in the Caspian Sea off Iran, except for sturgeons, but
during the Iran-Iraq War economic necessity brought back gill netting
for bony fishes and cooperatives were established (Habibnejaad, 1993).
Gill netter cooperatives were changed to kilka or beach seiner
cooperatives and by the end of 1993 no gill nets were allowed in the
Caspian Sea. However it took 12 years to overcome objections to
banning gill nets by fishermen and in parliament. Problems with excess
mortality through inappropriate fishing methods are not new. In the
period 1925-1930 the total length of long-lines used in the Caspian
Sea was 7-8,000 km while sturgeon nets exceeded 10,000 km. Many fish
died in unattended nets or tore lose from long-lines, later to die
from hook injuries (Sternin and Doré, 1993). The prohibition of the use of gill
nets with a less than 12 cm mesh in 1994 by Iran has conserved stocks along the
southern coast of the Caspian. Additionally licenses were restricted and fishing
co-operatives closed down in order to control the take (Raymakers, 2002).
Iranian fisheries have taken place mainly in the sea and so a lot of immature
fish are caught whereas the former Soviet fisheries took place in rivers where
only adults were taken (and ideally could be controlled more easily). However
state control in Iran has meant, as noted above, better control over the
fisheries and more effective conservation, although poaching does occur.
An attempt has been made to raise sevryuga sturgeon in the central Iranian desert
100 km southeast of Yazd (www.iranmania.com, downloaded 13 March 2003 and other
news reports) in a 5000 sq m artificial pond, perhaps more an indication of the
desperate straits of the sturgeon populations than anything else.
Pourkazemi et al. (2000) examined the phylogenetic relationships of
the 5 sturgeon species in Iran using mtDNA. Huso huso and Acipenser
nudiventris showed a close evolutionary relationship as did A.
gueldenstaedtii and A. persicus. The latter two species apparently
diverged about 1 MYA. Birstein and DeSalle (1998) using molecular techniques
found that the Ponto-Caspian species of sturgeons dispersed through the Black,
Azov, Mediterranean and Aral seas during the Pleistocene 1.5 MYA and later, the A.
stellatus-A. persicus lineage originated 6.0-5.5 MYA in the Upper
Miocene-Lower Pliocene, the A. gueldenstaedtii lineage and the Ponto-Caspian
sturgeons originated 15 MYA in the Middle Miocene, Acipenser originated
and diverged 95-65 MYA in the Upper Cretaceous, and Acipenseridae diverged from Polyodontidae, a related family, 200-135 MYA in the Jurassic.
An important, recent literature source on Caspian
sturgeons is Holčík (1989) as well as specific works on Iranian sturgeon biology and
fisheries by Rostami (1961b), Vladykov (1964) and Mobayen (1968). The fisheries
information in these last three works, relating to techniques and
stations, is somewhat dated and not detailed here (Raymakers (2002) gives a map
showing Iranian fisheries stations). A general account
of sturgeons is given in Birstein et al. (1997) and in Hochleithner and
Gessner (1999). Billard (2000) is a recent review of reproduction and
associated methodologies used on fish farms. CITES (2001) gives an
identification guide in English, French and Spanish, with numerous pictures and
diagrams. Pavlov et al. (2001) review
the types of spawning migrations carried out by sturgeons. Ruban and
Kohodorevskaya (2011) give an historic overview of the Caspian sturgeon
fisheries. There are numerous
other popular reports and scientific papers on Caspian Sea sturgeons, not all of
which can be cited or analysed here. A Bibliography of Sturgeons is given by Y. Keivany and V. J. Birstein at www.geocities.com/keivany/sturgbibl.html. Various
manuscript reports on the biology and rearing of the economically important
sturgeons have appeared in Farsi, e.g. Abdolhay (1997), Abdolhay and Baradaran
Tahori (1998), Baradaran and Abtahee (1998), Fadaee (1997), Kohneshahri and
Azari (1974), Moghim et al. (1996), Nasrichari (1993), Pourkazemi (1996),
Shafizadeh and Vahabi (1996), etc. Regular symposia on sturgeons are held and
extensive presentations and publications result, e.g. The 5th International
Symposium on Sturgeons, papers from it being published in Journal of Applied
Ichthyology 22(s1)(2006). These works have not all been summarised here because
of expense in obtaining copies and because many papers refer to details of
aquaculture physiology and biochemistry.
A general Farsi name for these sturgeons is سگ ماهي (sag mahi = dog fish).
Caviar
خاویار
Further information on the catches of sturgeons and
production of caviar in Iran can be found in the Species Accounts below.
Iran is the second largest producer of caviar, after Russia, with 20% of the
world market valued in excess of $50 million (Khajehpour-Khouei, 2000). Azari
Takami et al. (1997b) outline the historical development of the caviar
trade and Hosseini Seyed et al. (2008) ranks export goal markets for this
commercially important product.Sturgeon roe or eggs are known as caviar and form an expensive
delicacy (Bolourchi, 1997). The word caviar may come from Farsi "kaya-dar",
"khay-dar" or "khay-var" meaning "having eggs",
from خاگآور or khāgāvar for roe-generator,
or from "chav-jar" meaning "a cake of strength or power" or "bread of
lovers" in allusion to its reputed aphrodisiac qualities; havyar
in Turkish means "fish eggs" but the origin of this word seems in some
dispute among etymologists and it may be Greek (Georgacas, 1978; Sternin and Doré, 1993; Bolourchi, 1997).
In addition to sturgeon roe, eggs of other species are eaten in Gilan and
Mazandaran, where the meal is known as ashpal. The species include Rutilus
frisii, Abramis brama, Salmo caspius and less commonly
Cyprinus carpio and Barbus sensu lato spp. The roe can be eaten cured as a
condiment or when fresh, grilled, steamed or mixed with eggs and fried to form
ashpal kuku, a custard-like dish.
The history of the caviar industry in Iran is a complex subject,
variously reported in the popular media and in legend. The Russians
are said to have obtained writs from Moslem leaders in the Caucasus in
the early nineteenth century to the effect that Moslems could not eat
these fishes, leaving the valuable caviar fisheries for Russian
fishermen to monopolise (Kayhan International, 1 December 1962). The
caviar industry was first granted by the Iranian government to Stepan
Martinovitch Lianozoff (or Lionosoff, Lianozov) an Armenian subject of Czarist
Russia in 1873 (or 1876 or 1879, accounts vary), regularly renewed and later transferred to his only
son George. In 1896 the lease was renewed at an annual cost of 450,000 gold
francs (Fortescue, 1920). In one version of events, Martin (the grandson of Stepan)
disappeared in 1923, kidnapped while meeting two ravishing Armenian
sisters, leaving only a letter ceding his rights in the caviar fishery
to the Soviets (Tehran Mossavar Magazine, 18 April 1952; Time, 9
February 1953; L'Illustré, Lausanne, 20 January 1955; Tehran Radio, 6
May 1959). Another version simply has Martin selling his rights to the Soviet
Government (Mirfendereski, 2000) or refusing to pay during the vicissitudes of
the Russian Revolution. In 1919 another Russian subject, Grigor Petrovic
Vanitsof rented the southern Caspian fisheries for 20 years but could not
fulfill his obligations. A joint Irano-Soviet company, "Mahi Iran", formed
under Soviet pressure on the Iranian government, was given a monopoly
of the foreign sale of caviar in 1927 (in 1923 the fisheries of Astara,
Anzali and Hasan Kiadeh had been occupied by Soviet troops and
declared part of the Soviet fisheries). The Irano-Soviet company was
run almost entirely by Soviet technicians and the caviar was marketed
as of Russian origin (Kayhan International, 27 June 1959; Saffron, 2002). One part of the
Persia/U.S.S.R. agreement banned chemical and explosive uses for capturing fish
(Mirfendereski, 2000). The fishery was nationalised in 1953 and administered by the Iranian Fishery
Company (Sherkat Shilat). Most of the catch was sold to the former
U.S.S.R. (Anonymous, 1961b) and Soviet scientists organised caviar
production until the Iranian Revolution in 1979-1980 (Taylor, 1997).
Greenspan (1989) details more recent skullduggery.
Keyvanfar (1988) described the preparation of Iranian caviar from
the various species and the following is taken from that account.
Emadi (1994) and De Meulenaer and Raymakers (1996) also give accounts
of this process and the kinds of caviar obtained. Sturgeons are alive
or very fresh when brought to the processing plant. They are usually killed by a
blow to the head. Sex is determined with an awl-shaped instrument inserted into
the cloaca, pulling out some ova. The female is
split open along the belly and the eggs and the enveloping adipose and
connective tissues removed. The eggs are generally about 10% of the
body weight. However, an average beluga of 68 kg can yield 18 kg of
caviar in Iran (ca. 26%) (V. D. Vladykov, in litt., 1966), and
a 40 kg beluga from Iran yielded 8 kg of caviar (20%) (L'Illustré,
Lausanne, 20 January 1955). The largest amount obtained from a beluga
was 360 kg of caviar (V. D. Vladykov, in litt., 1966). The
other species give an average of 6 kg of caviar in Iran. The eggs are
separated from the tissues by breaking the ovaries into pieces by hand
and delicately pressing the eggs through a 10 x 10 mm screen. This
takes only a few minutes. The eggs are then washed in fresh, cold
(8-12°C) water for 30-40 seconds to remove fragments of ovarian tissue. The eggs are separated from the
washing water by collecting them on a very fine mesh screen, the
process taking 3-4 minutes. This type of washing is not done with A.
stellatus because of the fragility of the egg membrane in this species.
The type and quality of the caviar is determined next and they
depend on the colour, diameter and membrane strength of the egg. Large
eggs with a strong membrane and a clear, grey, dark brown or gold
colour are the best and are packed in metal containers. Small eggs
with fragile membranes and sombre colouring are second quality and
used for pressed caviar or bulk caviar. Pasteurised caviar is made
from eggs with weak membranes since the heat solidifies the membranes.
Salt is added at a rate of 4-6% to the weight of the eggs, varying
with the season and being higher in the warm summer months. The salt
is 99.2% sodium chloride and only 1-10 kg of eggs can be salted at one
time so that salting is uniform. Boric acid and borax are added in a
ratio of 2:3, comprising 20% of the total salt added, to aid in
conserving the caviar. Caviar for export to the U.S.A. is exempt from
this addition of boric acid and borax and only salt is used, 100 g for
each 1 kg of caviar. The salt is mixed delicately with the eggs by
hand for 50-250 seconds. A good salting process is essential for the
preparation of caviar and is evidenced by the eggs having small white
lines on their surface, the membrane becomes stronger and more
resistant, the egg proteins become denser and coagulate, the eggs lose
their adhesiveness, liquid stops coming from the eggs, and the density
of the brine coming from the process increases. When these factors are
detected the salting process is stopped. A salting process which is
too long removes too much protein from the eggs and causes the eggs to
clump together. A process which is too short removes too little water
from the eggs and these eggs lose water gradually over several days in
their container and become soft and semi-liquid. The eggs are then
separated from the brine on a very fine mesh screen.
The U.S. Customs Service produces a description of caviar for the
trade community (www.customs.ustreas.gov/imp-exp1/comply/caviar.htm
downloaded on 20 July 1999). Caviar is graded on grain size, colour,
flavour and firmness. Gold coloured caviar is the rarest and most
desirable followed by light grey. Large grains are preferred over smaller ones.
There is a demand for caviar without borax and boric acid and such
chemicals as methyl parahydroxy benzoate and propyl parahydroxy
benzoate have been examined in Iran as alternatives (Iranian
Fisheries Research and Training Organization Newsletter, 7:3, 1995).
Fresh caviar is not salted and requires careful refrigeration; its
shelf life is short, a maximum of six weeks. Lightly salted caviar is
called "malossol" from the Russian for "little
salt", usually a 2.4% content in Iranian caviar which is very
good quality compared to some caviar which contains up to 11% salt.
The higher the salt content the longer the shelf life. Chilled
malossol kept at -2 to 4°C will be edible
for up to three months. Pressed caviar is prepared in a similar fashion except the salt
content is higher, at 7% in the finished product. It will keep for a
long time at 4-8°C. Borax gives a longer shelf life too and is less
dangerous to human health than the amount of salt needed to give the
caviar an acceptable shelf life. Most caviar consumed world-wide is
pasteurised as some countries do not accept caviar with borax and
higher salt levels are not acceptable to consumers. Pasteurised caviar
has a shelf life of 12-15 months (De Meulenaer and Raymakers, 1996). Caviar
should not be frozen or pasteurised as this affects the taste.
Good quality caviar must be refrigerated. U.S. packaged caviar also
contained tragacanth gum according to labels on jars from the 1960s. Bankehsaz
(2009) found that the quality and grade of exported caviar can be maintained if
storage time is less than 6 months in a -3°C cold room. Razavilar et al.
(2001) found Iranian caviar to have a good microbial condition during processing
and storage in Mazandaran.
The caviar is placed in boxes of 0.5 to 2 kg, each box being filled
to within 1-2 cm of the lid. Sternin and Doré (1993) give tin sizes
of 0.6 and 1.8 kg with a limited amount of 100, 200 and 300 g tins -
most caviar is repacked at its destination in 30 g, 50 g, 125 g, 250
g, 500 g and 1 kg tins and jars). The lid is pressed on centrally to
exclude as much air as possible and the excess brine is allowed to
drain away by stacking the boxes vertically for 1-15 minutes. One
further press is carried out manually, the outside of the box is
cleaned, and boxes are stacked in piles of five for 20-24 hours in the
cold season (October-March) and 12 hours in the warm season. During
this period, the pile of boxes is turned over several times to remove
the last traces of excess brine. After one last press on the centre of
each box to ensure the lid adheres to the eggs and no air remains, the
box is sealed hermetically with a ring of rubber. Well-prepared caviar
has lost 4-6% of its initial weight, has a salt content of 3-5% and
the eggs are separate and non-adhesive. Caviar in this form will keep
for a long time at 0-2°C. Caviar is re-packed in fully airtight tins,
slowing down the maturation process for three months after which the caviar
deteriorates.
A microbiological analysis of Iranian caviar imported to Turkey has been
carried out by Altug and Bayrak (2003) who did not find any pathogenic and toxin
producing Salmonella spp. and Clostridium perfringens. Coliforms,
bacteria and yeasts showed some high counts, perhaps contamination during
production stages.
First quality caviar consists of healthy, non-fragile eggs from one
species with a large or medium size. The caviar is dry, of uniform
colour - between clear-grey and dark-grey - without odour or abnormal
taste. The box is filled within a centimetre of the edge. Second
quality caviar has eggs which may be fragile, are of large, medium or
small size, their colour varies from clear-grey to black, and they may
be damp. Yellowish or brown caviar from A. gueldenstaedtii is
acceptable for these two qualities of caviar. Egg size is determined
by the cubic centimetres occupied by 100 eggs, e.g. for A.
gueldenstaedtii large eggs occupy >1.9 cc, medium 1.4-1.9 cc
and small <1.4 cc and for A. stellatus large eggs occupy
>1.3 cc, medium 0.9-1.3 cc and small <0.9 cc. Egg sizes are not
determined for Huso huso and A. nudiventris. Eggs of the
former are much larger than A. gueldenstaedtii eggs while those
of the latter are nearly the same size as A. stellatus eggs.
.jpg)
Russian and Iranian caviar tins, beluga, osetra and sevruga (Wikimedia Commons)
Sturgeon species cannot be readily identified from the size or
colour of the eggs making up caviar. Diet, pigmentation of the adult,
and age of the fish all appear to influence egg colour. Huso huso
eggs are often light to dark grey, Acipenser gueldenstaedtii
eggs are blackish to brown or almost golden and A. stellatus
eggs are black according to traders. White caviar where the egg has a
red spot on it is from albino fish. Light grey beluga and light yellow
oscietra caviar are now very rare, in the past being found in only a
small proportion of the species population which itself is now in
decline (De Meulenaer and Raymakers, 1996). Le Comptoir du Caviar,
which markets caviar (www.gourmet-tradition.com/en/comptoir_du_caviar.html,
downloaded on 19 March 1999), describes Iranian sevruga as grey to
black with fine grains and an iodised taste, Iranian oscietre as grey-black
with bronze shades, middle-sized grains, very iodised with a little
taste of walnut, and Iranian beluga as very rare, dark or light grey,
large grains and a fine and gusty savour.
The single biggest market for caviar is first-class airline
passengers. Supermarkets, hotels, restaurants and specialised
retailers also market caviar. France consumed the largest amount in
the 1990s, about 60-80 t, while Germany consumed 40-50 t. The Shilat
packages its product carefully to ensure consumers know the caviar is
genuinely Iranian. The large tins in which the caviar is packed keep
their contents edible for 12-18 months at -2 to -3°C
(the oil content and added salt prevent freezing). These tins are
sealed in a piece of net which in turn is sealed on both sides with
consecutive numbers, placed in a sealed linen bag and then in a wooden
box. Each tin is also marked with the loading station number (where
the fish are brought after capture to have their caviar removed) and
also the number of the individual fish scratched on the side. Tins are
shipped by air in "cooltainers" which have their own
refrigeration unit. These large tins are vacuum-packed into smaller
ones in packing centres in Europe (Christie, 1995). The main market in
2000 was Japan to which 30% of Iranian production was exported. Permanent markets in
Europe are Switzerland, Germany, France, Luxembourg and Spain (I.F.R.O.
Newsletter, 26:3, 2001) and the European
Union is often the biggest importer of Iranian caviar. Iran was the top exporter of caviar in
the year 2000 at 71.5 t valued at $34.4 million (IFRO Newsletter,
28:2, 2001). This is a value increase of 17% although the amount was less than in 1999 at
84.9 t. In 2002, 87% of caviar came from Iran
(www.caviar.ru/english/digest.htm, downloaded 12 December 2002) although IRNA (8 December 2002) gives a
figure of of almost 50%. The caviar export quota was 50,505 kg for Iran in 2006 (iran-daily.com, downloaded 28 July 2006)
or 44.3 t (Iran Daily, 11 September 2006).
Iranian caviar sold in major airports like Heathrow in London comes
in several kinds. Caviar House markets imperial, which has large gold
grains and was previously reserved for the Shah's family (from Acipenser
persicus); beluga, light to dark grey and large grained; royal
black consisting of large deep-black grains from a 20-40 year old
osetr; "oscietre", which is dark grey-brown to a golden
yellowish; classic grey, a pale grey with large grains; and sevryuga,
which is dark grey and fine grained. Prices vary with quality and time
as shown below (personal observations):-
|
|
Imperial |
Beluga |
Royal Black |
Oscietre |
Classic Grey |
Sevryuga |
|
December 1993 |
|
|
|
|
|
|
|
50 g |
£82 |
£76 |
£48 |
£36 |
£38 |
£23 |
|
1000 g |
£1411 |
£1318 |
£819 |
£621 |
£656 |
£399 |
|
September 1995 |
|
|
|
|
|
|
|
50 g |
£94 |
£101 |
£54 |
£48 |
£40 |
£36 |
|
1000 g |
£1640 |
£1759 |
£939 |
£836 |
£697 |
£630 |
|
September 1997 |
|
|
|
|
|
|
|
50 g |
£89 |
£88 |
£53 |
£47 |
£39 |
£34 |
|
1000 g |
£1536 |
£1540 |
£912 |
£812 |
£680 |
£590 |
|
November 1999 |
|
|
|
|
|
|
|
50 g |
£140 |
£160 |
£75 |
£60 |
£65 |
£53 |
|
1000 g |
£2420 |
£2770 |
£1312 |
£1060 |
£1138 |
£920 |
|
November 2000 |
|
|
|
|
|
|
|
50 g |
£184 |
£208 |
£114 |
£95 |
£99 |
£79 |
|
1000 g |
£3541 |
£3987 |
£2177 |
£1818 |
£1894 |
£1527 |
| April
2002 |
|
|
|
|
|
|
| 50 g |
£184 |
£208 |
£114 |
£95 |
£99 |
£79 |
| 1000 g |
not given |
not given |
not given |
not given |
not given |
not
given |
The types of caviar listed changed in 2003 as follows:-
|
|
Imperial
XO
|
Beluga |
Beluga XXL |
Royal Black |
Royal Black XL |
Oscietre Gold |
Classic Grey |
|
March 2003 |
|
|
|
|
|
|
|
|
50 g |
£289 |
£160 |
£309 |
£11 6 |
£197 |
£119 |
£125
|
The types of caviar listed changed in 2004 as follows:-
|
|
Imperial
XO
|
Beluga |
Beluga XXL |
Royal Black |
Royal Black XL |
Classic Grey |
Sevruga |
Oscietre |
Imperial |
|
September 2004 |
|
|
|
|
|
|
|
|
|
| 50 g |
£145 |
£160 |
£195 |
£88 |
£114 |
£66 |
£56 |
£75 |
£98 |
| 100 g |
£275 |
£318 |
£385 |
£175 |
£225 |
£129 |
£110 |
£149 |
£195 |
| 200 g |
£540 |
£619 |
£750 |
£340 |
£435 |
£247 |
£221 |
£297 |
£385 |
The types and weights of caviar listed changed in 2006 as follows for
"Prestige Selection":-
|
|
Beluga |
Royal Black |
Classic Grey |
Sevruga |
Oscietre |
Oscietre Gold* |
|
March 2006 |
|
|
|
|
|
|
| 50 g |
£155 |
£125 |
£105 |
£95 |
£120 |
£130 |
| 125 g |
£380 |
£305 |
£255 |
£230 |
£295 |
£320 |
| 250 g |
£750 |
£595 |
£495 |
£450 |
£580 |
£630 |
* = golden-coloured eggs from a mature oscietre.
The types and weights of caviar listed changed in 2006 as follows for "Prunier":-
|
|
Traditional |
Saint James |
Great American |
Paris |
Heritage |
|
March 2006 |
|
|
|
|
|
| 50 g |
£65 |
£85 |
£95 |
£120 |
£155 |
| 125 g |
£155 |
£205 |
£230 |
£295 |
£380 |
| 250 g |
£305 |
£405 |
£455 |
£585 |
£755 |
| 500 g |
£605 |
£805 |
£905 |
£1165 |
£1505 |
| March 2007 (500 g not listed) |
|
|
|
|
|
| 50 g |
£75 |
£110 |
(not listed) |
£120 |
£170 |
| 125 g |
£185 |
£270 |
(not listed) |
£295 |
£415 |
| 250 g |
£370 |
£535 |
(not listed) |
£585 |
£830 |
The types and weights of caviar listed changed in 2006 as follows for
"Private Reserve":-
|
|
Royal Black XL |
Imperial XO |
Beluga XXL |
|
March 2006 |
|
|
|
| 50 g |
£135 |
£145 |
£195 |
| 125 g |
£330 |
£335 |
£480 |
| 250 g |
£650 |
£695 |
£950 |
Prices for these brands were not listed in March 2007, and were only
available on request, indicative of both scarcity and constantly changing
prices. Names of the different types of caviar keep changing so it is
difficult to track price increases. In November 2008, beluga caviar from
Caviar House was selling for
£2170 for 250 g and other types had also continued to increase in price,
often dramatically. Curiously, in July 2010 under "Caspian Sea caviar"
beluga was selling at
£1340 per 250 g. In 2011, all available caviar was Russian or farmed. In
2012, prices were
for 250 g of Beluga and Imperial caviar £1650 and for Royal Black and
Oscietre £790.
Taylor (1997) gives prices in Deutschmarks (DM) per kilogramme net
weight (no duty paid) for Iranian caviar over 12 years including the
approximate "bazaar" or illegal price for smuggled caviar
(note also that A. gueldenstaedti probably includes A.
persicus):-
|
Year |
Huso huso |
A. gueldenstaedti |
A. stellatus |
"Bazaar" |
|
1983 |
540 |
408 |
341 |
200 |
|
1984 |
600 |
424 |
400 |
180 |
|
1985 |
675 |
465 |
404 |
180 |
|
1986 |
650 |
460 |
345 |
200 |
|
1987 |
650 |
414 |
325 |
180 |
|
1988 |
1630 |
445 |
310 |
180 |
|
1989 |
2600 |
510 |
345 |
220 |
|
1990 |
1596 |
432 |
304 |
220 |
|
1991 |
1600 |
450 |
337 |
180 |
|
1992 |
1600 |
470 |
345 |
160 |
|
1993 |
950 |
435 |
345 |
160 |
|
1994 |
950 |
500 |
355 |
80 |
Taylor (1997) also compares demand from western markets with supply
from Iran; for Huso huso demand is 0.2 tonnes while supply is
2.0 t, for A. gueldenstaedti type I.A 2.0 t and 0.5 t, for A.
gueldenstaedti type I.B 60.0 t and 40.0 t, for A.
gueldenstaedti type II 15.0 t and 10.0 t, for A. stellatus
I 100.0 t and 30.0 t, and for A. stellatus type II 100.0 t and 25.0 t.
Friedland (1986) gives a variety of recipes for caviar dishes.
Rehbein (1985) and Keyvanfar et al. (1987) studied soluble caviar proteins of
sturgeon species including A. gueldenstaedtii, A. stellatus, A. nudiventris and Huso
huso. They were able to distinguish the species on this basis and
thus provide a means of detecting fraudulent caviar. Rezvani Gilkolaei (2002)
used DNA PCR amplification and RAPD markers to identify caviar, in particular
that of the endangered Acipenser nudiventris whose caviar has been
substituted for more expensive caviar of A. gueldenstaedtii and A.
persicus. Rehbein et al.
(2008) tested and reviewed different methods for identifying caviar by species,
including DNA, differential scanning colorimetry and determination of stable
isotopes. Gessner et al. (2008) were able to distinguish between farmed
and wild sturgeons based on fatty acid composition and recommend use of specific
fatty acids as additives in the formulated diets of farmed fish. However, Ludwig
(2008) reviewed methods of identifying caviar and other sturgeon products and
detailed difficulties. No single method met the criteria he established
(species-level identification, population identification, wild versus
aquaculture, age of caviar). Cost was also a factor. Keyvanfar (1984)
was unable to find genetic polymorphism in erythrocytes of the four
species listed above using serological techniques.
Moghim et al. (2012) isolated and characterised microsatellite loci to
assist in identifying population structure of sturgeon species for use in
conservation work.
Genus Acipenser
Linnaeus, 1758
This genus is characterised by a small, transverse mouth (large and
crescentic in Huso), by the gill membranes being joined to the
isthmus and not to each other (joined to each other and free of the
isthmus in Huso), by a rounded or elongate snout, and cylindrical barbels.
Bani et al. (2008) give details of brain morphology in Acipenser
stellatus and A. persicus that suggest sturgeons have evolved
different sensory strategies to cope with life in the deep sea.
There are 16 species in the genus and 4 are reported from Iran.

Ventral view of heads of Huso huso,
Acipenser nudiventris, A. gueldenstaedtii
and A. stellatus
(A. persicus is similar to A. gueldenstaedtii)
Acipenser baerii
Brandt, 1869
Introduced to the Caspian Sea basin by Soviet authorities (Karpevich
and Lukonina, 1971; 1972; McNeil, 1979) but no records from Iran.
Acipenser gueldenstaedtii
Brandt and Ratzeburg, 1833

Common names
چالباش (= chalbash or short head),
تاس ماهي (= tas mahi or bald fish; this term includes A.
gueldenstaedtii, A. persicus and A. nudiventris for large eggs, in
fisheries statistics), تاس ماهي روس (= tasmahi-ye Rus
or tasmahi-e-russ), تاس ماهي ايراني
(= tas mahi Irani), osiotra, osyetra, سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), kaviari rusi.
[russkii osetr or Russian sturgeon in Russian; nere or rus neresi in Azerbaijanian; bekra or bekre balyk in Turkmenian].
Systematics
This species was originally described in part from the Volga, Ural
and Terek rivers of the Caspian Sea. Sometimes spelt güldenstädti,
but accents on letters are not used in Latin scientific names.
Birstein et al. (1997) and Reshetnikov et al. (1997)
spell the name gueldenstaedtii, regarding the double "i"
ending correct as opposed to the emended single "i" which
appears in much recent literature.
Acipenser gueldenstaedti persicus natio kurensis Belyaeff, 1932 was described as the
Kura River subspecies but see below under Acipenser persicus.
Comparison of serum proteins have shown antigenic characteristics
distinguishing Volga and Kura River stocks in the Caspian Sea, matched by morphometric characters.
The fishes identified as A. gueldenstaedtii in Iran may well
be almost entirely A. persicus, although this remains to be
determined. Consequently data on morphology and biology are confused in many accounts.
The distinction of A. persicus is questioned by authors (see below).
Some specimens have strong spines on the scutes and have been
described as morpha aculeatus Lovetzky, 1834 although this has no nomenclatural status.
Birstein and Ruban (2004) and Birstein et al. (2005) state that this species has at least three,
morphologically indistinguishable, genetic forms in the Caspian Sea. These are the pure form, one similar to A. baerii
of Siberia, and one to A. naccarii of the Adriatic, with competing
hypotheses to explain this. The most likely hypothesis is that the Caspian forms
are closely related to the ancestral forms of the three species, evolving first
as subdivisions of the original Caspian Sea population and then moving to
different geographical areas when the Caspian was connected to them.
Pourkazemi et al. (1999; Rezvani Gilkolaei, 2000) found two distinctive genotypes and therefore
populations of A. gueldenstaedtii in Iranian waters using
molecular techniques. This species and A. persicus showed great degrees of similarity in a phylogenetic
analysis (Iranian Fisheries Research and Training Organization
Newsletter, 14:4-5, 1996; Pourkazemi et al., 2000). The common origin of the two species
was about 1 million years ago (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 61-62, 1997; Pourkazemi et al., 2000).
Key characters
This sturgeon has a short snout (less than 60% of head length) with
a rounded tip in contrast to the long snout (>60%) and pointed tip
in A. stellatus. Huso huso has an unusual,
crescent-shaped mouth and continuous gill membranes forming a fold on
the isthmus and A. nudiventris has a continuous lower lip and
usually more than 50 lateral scutes. Closely resembling A. persicus,
it is distinguished from that species by the short and blunt snout,
yellowish-white belly and golden-brown back. A drawing in Vlasenko,
Pavlov and Vasil'ev in Holčík (1989) of the two species has a snout length in head length of 4.3 as
opposed to 3.2 for Acipenser persicus but figures of snout
length in total length overlap for the two species. The interorbital
distance is much less in the Persian sturgeon (29.2-30.5% of head
length) than in A. gueldenstaedtii (Artyukhin and Zarkua, 1986)
but in small specimens examined by me from Iran, some had gueldenstaedtii
interorbital distance and persicus snout length. There is a
colour plate and line drawings of the heads of the two species from
the Black Sea in Birstein et al. (1997:8, 220).
Morphology
The lower lip is interrupted at its centre. Barbels are not
fringed, lie nearer the snout tip than the mouth, and do not extend
back to the mouth. Sheibani (2003b) described the posterior alimentary canal in
this species.
Dorsal fin rays 26-51, anal fin rays 18-35. Gill rakers 15-36.
Dorsal scutes 5-19, lateral scutes 21-50 and ventral scutes 6-14.
There are rows of smaller star-shaped scutes between the dorsal and
ventral rows in some fish, rounded in this species and more triangular in A.
persicus. The chromosome number is 2n=250 ± 8, 2n=249 ± 2, 2n=247 ± 7 or
2n=258 ± 4(Klinkhardt
et al., 1995; Havelka et al., 2011).
Sexual dimorphism
Females are larger than males of the same age.
Colour
The back is usually golden-brown but may be olive-grey to dark
green, the flanks grey-brown, and the belly yellowish-white or rarely
a lemon yellow. Young are blue dorsally and white ventrally.
Size
Attains 160 kg and 2.36 m, perhaps as much as 4 m although not confirmed (Machacek
(1983-2012), downloaded 27 July 2012). In Iran,
fish identified as this species (see Systematics) averaged
16-20 kg and 1.4-1.6 m in the 1950s (Farid-Pak, no date). Tsepkin and
Sokolov (1971) state that Safid River fish reach 2.42 m. De Meulenaer
and Raymakers (1996) give 200 kg and an average length of 2 m.
Distribution
This species is found in the Caspian Sea, particularly in the Volga
River basin, as far as Moscow in the past. Very small numbers are
caught in the Kura and Astara rivers. Also found in the Black Sea
basin. Khodorevskaya et al. (2001) review abundance and distribution in former
Soviet waters of the Caspian Sea. It is less common than Acipenser persicus
in Iranian waters.
In Iran, it is recorded from the Astara River in the west to the Gorgan
River in the east (but see Systematics). Reported recently from
such rivers as the Atrak, Gorgan, Gharasu, Tajan, Babol, Haraz, and
Safid, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea (Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli and Naderi, 2009). V. D. Vladykov's field notes in the early
1960s reported it from Kopurchal, Khadjenafas, Tazeabad, 12 Bahman, Nevissi,
Izadeh and Hasan Kiadeh. Access to many rivers must now be
restricted by reduced water flow, construction, weirs, dams, irrigation canals and pollution.
Zoogeography
Presumably a relict of the isolation of the Caspian and Black seas
from the Mediterranean-Atlantic.
Habitat
There is no marked seasonal variation in depth distribution in the
south Caspian Sea in contrast to the middle Caspian. This species is
found over sand or sandy-silt bottoms in a temperature range of
2.3-24.8°C and a salinity range of 6.28-14.34‰ in the sea. Farabi et al.
(2011) examined experimentally salinity tolerance in juveniles and found it, and
survival, to increase with age. It approaches closer
to the coast in winter (February) than other sturgeons because it
favours colder temperatures. It is found in numbers down to 50 m with
only the occasional specimen being caught below this depth (Legeza,
1972; 1973). Brackish water is favoured because of food
concentrations. High oxygen concentrations are needed, 6-7 mg/l for
adults, although larvae only require a minimum of 1.56 mg/l at 20°C.
Reproduction in the Kura ceases when temperatures reach 26°C.
Eggs are sensitive to oil concentrations of 0.5-1.0 mg/l. Levin (1997)
reports concentrations on the western shelf of the Caspian Sea during
winter, as far south as Azerbaijan, at depths of 5-24 m.
Age and growth
Veshchev and Novikova (1986) and others have recently studied the
spawning run of this species in the Volga River and found fish from 7
to 39 years old with 87.9% 13 to 27 years old. The spawning population
comprises 32 age groups therefore. Males dominate at 63.6%. Males vary
in length from 101 to 185 cm and weigh 3 to 38 kg and females from 116
to 200 cm and 9 to 46 kg. Most males begin to reproduce at 11-13 years
while females begin at 12-16 years. Growth can be rapid,
young-of-the-year reaching as much as 35 cm by autumn. Life span
exceeded 48 years in the past. Khodorevskaya et al. (1993)
cited in Levin (1997) gives Volga River spawning ages of 8 to 35 years
with females 6-8 years older than males. Females have an average
weight of 26-29 kg and a length of 136-163 cm; males are 12.0-14.5 kg
and 130-134 cm. Females mature at 10 years, 2-3 years later than males.
Minimum spawning intervals are 2-3 years for males and 3-4 years for females.
Von Bertalanffy growth parameters in Iranian females are L∞
= 201 cm and K = 0.073 or 192 cm and 0.082 and for males 189 cm and
0.092 depending on the methodology used. Total mortality (Z) was
0.33-0.67 for females and 0.46-0.82 for males, natural mortality (M)
was 0.05 for females and 0.06 for males, fishing mortality (F) was
0.62 for females and 0.39 for males, and optimum fishing mortality was
(F) 0.21 for females and 0.37 for males (Iranian Fisheries Research
and Training Organization Newsletter, 16:4-5, 1997).
Food
This species is primarily a mollusc eater (Polyaninova et al., 1999) but also takes
crustaceans such as chironomids and gammarids and small fishes such as
gobies (Gobiidae) and Clupeonella caspia. The introduced
species of mollusc, Abra ovata, polychaete, Nereis
diversicolor, and crab, Rhithropanopeus harrisii are now
important diet items at 49.3%, 12.3% and 9.2% respectively in the
Caspian Sea. The importance of oligochaetes like Nereis
and Enchytraeus albidus in the diet of sturgeon species is
recognised in Iran and studies on their ecology have been carried
(IFRO Newsletter, 28:3, 2001).
In rivers, fingerlings feed on various benthic organisms. Hajimoradloo et al.
(2002) examined the diet of juvenile fish taken in beach seines from the
Miankaleh peninsula in Golestan and compared it with the diet of A.
persicus. The latter favoured cumaceans while A. gueldenstaedtii
favoured gammarids. Both species had more empty stomachs in autumn and less in
winter. A. gueldenstaedtii had more empty stomachs in all seasons than
A. persicus. The food niche width was less in A. gueldenstaedtii and
food overlap was highest in winter and lowest in spring.
Reproduction
Spawning migrations in sturgeons are triggered by temperature,
daylength and flood discharge. This has been discussed more fully by
Barannikova (1972) along with the effects of dams on this complexly
timed, hormonal process. In northern rivers the water temperatures are
8-18°C (Artyukhin and Zarkua, 1986). The adult loses 25-30% of its weight
after spawning and females are only ready to spawn again after 4-6
years and males after 2-4 years.
The spawning run in the Kura River is complex and four
"races" have been recognised (Gerbilskii, 1955; Berg, 1959).
These are early and late vernal, spawning in their year of entry, and
summer-arriving and autumn-arriving hiemal which overwinter to spawn
the following spring. The chief spawning period in the Kura River is
from the end of May to the beginning of July (Zakharyan, 1972). (Note
that this may in fact apply to A. persicus).
In the Volga River, A. gueldenstaedtii has a run beginning at the
end of March or beginning of April at 1-4°C,
peaking in July. Migration speed in the Volga is 18.1-22.6 km/day.
Eggs are laid on gravel or stone beds at 4-25 m depths and a current
velocity of 1-1.5 m/sec. in the Volga River. Some eggs are laid in
shallower, flooded areas. Egg incubation is optimal at 9-15°C.
The downstream migration of spawned out fish in the Volga begins in
the second half of May and peaks in June and July. Levin (1997)
summarises the migration of Volga River fish as follows. The small
population of the early spring race enters the Volga delta in
April-May and migrates upriver for 600-700 km before spawning in
May-June at 12-15°C. The late spring race migrates to spawning sites in May-June, spawning
in July-August at 19-22°C. In June-July the winter race enters the delta but only migrates
upriver in the next summer. In August-October the late winter race
enters the river. These winter races overwinter in deep parts of the
river and spawn in April-May at 9-13°C.
Volga River sturgeons had a fecundity of 332,900 eggs in one study
(Veshchev and Novikova, 1986), elsewhere reported up to 1,165,000 eggs
for the Volga. The Safid River sturgeon fecundity is said to be less
(this may be A. persicus). Eggs are brownish-grey and ovate, up
to 3.3 x 3.8 mm in dimensions. A 150 kg fish yielded 5 kg of caviar (IFRO
Newsletter, 29:4, 2001).
The sexual cycle lasts 2-3 years on the Iranian coast and is described by
light microscopy in Hedayatifard et al. (2009).
The caviar of this species comprises 4-5 kg on average, making up
16% of the body weight in Iran. In Mazandaran fish enter rivers in
autumn, overwinter and spawn in spring (Iranian Fisheries Research
and Training Organization Newsletter, 9:6, 1995).
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) describe Corynosoma caspicum as a
new species from this and other sturgeon species in Iran. Mokhayer and
Anwar (1973) report the following parasites from Iranian sturgeons in
general. These are the protozoan Trichodina reticulata, the
coelenterate Polypodium hydriforme, the trematodes Skrjabinopsolus
acipenserinus and S. skrjabini, the cestodes Amphilina
foliacea, Bothrimonus fallax and Eubothrium
acipenserinum, the adult nematodes Ascarophis ovotrichuria,
Cyclozone acipenserina and Cucullanus sphaerocephala,
the larval nematodes Contracaecum squalii, Anisakis
schupakowi and Eustrongylides excisus, the acanthocephalans
Leptorhynchoides plagicephalus, Pomphorhynchus laevis
and Corynosoma caspicum, the annelid Piscicola geometra
and the crustacean Pseudotracheliastes stellatus. Polypodium
hydriforme destroys the eggs of sturgeons, up to 80% of the
gonads, rendering reproduction insufficient to maintain the species. Amphilina
foliacea causes parasitic castration in sturgeons. Many of the
parasites provoke anaemia or block the intestine when numbers are
high. Pomphorhynchus laevis is capable of piercing the
intestine. Eustrongylides excisus produces stomach abscesses.
Ectoparasites take blood but also facilitate attack by bacteria and
fungi. On fish farms, Trichodina reticulata can cause high
mortalities while having no apparent effect under natural conditions.
Parasite numbers are controlled on fish farms by immersing the
sturgeons in salty water to remove ectoparasites, by feeding food
items known not to be carriers of parasites and avoiding such natural
foods and intermediate parasite hosts as amphipods. Mokhayer (1976b)
reports gas bubble disease in Iranian sturgeons without specifying the
species of sturgeon as well as the monogenetic trematodes Diclobothrium
armatum and Nitzschia sturionis, the digenetic trematodes Skrjabinopsolus
acipenseris and S. skrjabini, the cestodarian Amphilina
foliacea, the cestodes Bothrimonus fallax and Eubothrium
acipenserinum, the nematode larvae Anisakis schupakowi, Contracaecum
squalii and Eustrongylides excisus, and the nematode adults
Ascarophis ovothricuria, Cucullanus sphaerocephala and Cyclozone
acipenserina, the acanthocephalans Corynosoma caspicum, Leptorhynchoides
plagicephalus and Pomphorhynchus laevis, and the crustacean
Pseudotracheliastes stellatus.
Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in
juveniles at a frequency of 6.42%. Sattari et al. (2002) record
Cucullanus sphaerocephalus, Eustrongylides excisus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus,
Anisakis sp. and Corynosoma strumosum, the fauna being similar to
other sturgeons because of their piscivorous feeding. Hajimoradloo and Ghorbani Nasrabadi (2003)
found the prevalence of metazoan parasites in juveniles of this fish in the
southeast Caspian Sea to be 8 species with Anisakis larvae the highest at
13.3%. Pazooki and Masoumian (2004) report on blood parasites form fish caught
at Anzali, recording Cryptobia acipenseris and Haemogregarina
acipenseris. These parasites caused no pathological effects in the wild fish
but can lead to severe infections and cause anaemia on fish farms. Sattari and
Mokhayer (2005a; 2005b) recorded the occurrence of parasites in this species
from the Iranian southwestern and central coast of the Caspian Sea. The species
found were the nematodes Cucullanus sphaerocephalus, Eustrongyloides
excisus and Anisakis sp., the acanthocephalans Leptorhynchoides
plagicephalus and Corynosoma strumosum, and the digenean trematode
Skrjabinopsolus semiarmatus. General conclusions were that the diversity of
parasites was less in Iranian waters than in the northern Caspian Sea, perhaps a
reflection of the more varied habitat, its productivity and the carbonate ions
differing between the two regions. The diversity of parasite seems to have
declined over time also, perhaps as a result of unfavourable environmental
conditions, particularly in the freshwater ecosystem which limits the waters
available for spawning and parasite acquisition.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
A wide range of fish species are predators on the eggs of this
sturgeon and the young are taken by Silurus glanis, Alosa spp.,
Huso huso, and gobiids.
Economic importance
Chalbash have been fished in the Caspian Sea for at least 6000
years based on excavations at a Neolithic site on the eastern Caspian
coast (Tsepkin, 1986).
This particular species is fished primarily in the months of
September and in April-May in Iran. Caviar yield was 4-7 kg per female
in the 1950s (Farid-Pak, no date). Yields from 1963 to 1967 of meat
(and caviar) were 794.2 tonnes (69.3 tonnes), 918.2 (66.7), 849.0
(71.8), 974.6 (72.8), and 977.1 (75.9) respectively (RaLonde and
Walczak, 1970b). A commercial house maintains (1995) that this species
comprises 27% of the total catch. These data presumably include or are
almost entirely A. persicus in Iran. Spring-caught chalbash produce 2-3
kg of eggs per fish while those caught in the fall have egg weights of
3-4 kg. The former are more suitable for pressed caviar than the
higher priced grain caviar made from the larger eggs of fish caught in
fall (Vladykov, 1964). Figures for tas mahi (this species plus A.
persicus, and also A. nudiventris when eggs are large)
average yearly catches in Iran were given by Vladykov (1964) for the
period 1927/28-1931/31 to 1957/58-1961/62. Body weight varied from
264,105 kg (36.9% of total sturgeon catch) to 842,050 kg (78.9%) while
caviar weight varied from 33,098 kg (69.3%) to 159,931 kg (85.1%)
although the lowest percentage share of caviar for any of the
five-year periods in tas mahi was 28.6%. The category of tas mahi
provided the majority of eggs for caviar up to 1946/1947 (50-89%) but
this fell to 29-31% for the period after 1949/1950 in Vladykov's data.
Earlier data from Nevraev (1929) listed as A. gueldenstaedtii
and A. nudiventris combined for the Astara region of Iran gives
catches of 2002 to 9176 individuals for the period 1901-1902 to
1913-1914, for the Safid Rud region 26,721 to 54,257 individuals for
the period 1899-1900 to 1913-1914, for the Mazandaran region 4065 to
8818 individuals for 1906-1907 to 1913-1914, and for the Astrabad (=
Gorgan) region 2988 to 6044 individuals for 1902-1903 to 1913-1914. The capture
fishery for tas mahi (A. gueldenstaedtii, A. persicus and A.
nudiventris) was 89%, 4.2% and 6.2% respectively in 1973 but by 1993 had
changed to 27%, 69% and 4% due to fingerling production of A. persicus (Abdolhay
and Tahori, 1999). The stock of this species in Iranian waters in 2001 was 9.4
million (0.64 million) specimens comprising 12,900 tonnes (2074 t) with a
commercial stock of 220 t (223 t) (Ivanov and Kanunin, 2001; figures in
parentheses from text which does not agree with table).
Catches of this species in the southern Caspian Sea have declined from 837 t
and 602 kg/boat/day in 1971-1972 to 57 t and 0.34 kg/boat/day in 1999. Young
fish decreased in the decade prior to this study while older fish dominate at
present (Moghim, 2004a). A sharp decrease in sea ranching of fingerlings and a
consequent decrease in young fish abundance, will cause a a considerable decline
in future catches.
Dry-smoked flesh (balyk) is especially favoured in Russia
where this species occupies the first place in catches. Catches in the
period 1898-1913 in the northern Caspian reached 10,000 tonnes a year
only to decline through overfishing. The ban on sea fishing in 1941,
restricting catches to rivers where they could be more closely
controlled, led to a rebound of stocks and by 1977 a record catch of
11,980 tonnes was made.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food.
Conservation
Sturgeons generally are conserved by fish farming and release of
young and fry, attempting to augment natural populations. Stroganov
(1968) reviews Russian fish farming methods. Derzhavin (1923) reported
release of 7,620,000 fingerlings in the Safid River of Iran in 1923. Release
of unfed sturgeon fry was discontinued in Iran in 1965 as
unproductive. A hatchery produced annually 5.5 million sturgeon
juveniles at 3-5 g each (McNeil, 1979), comprising the species A.
stellatus and A. gueldenstaedtii (the latter presumably
includes A. persicus). The Sad-e-Sangar (Dr. Beheshti or Martyr
Beheshti) Fish Farm or Hatchery 27 km from Rasht in Gilan produced
14-15 million sturgeon larvae in 1987 and up to 3 million 2-3 g
sturgeon are produced annually (Petr, 1987). Fingerling production
from four hatcheries in Iran reached a record high of 12 million fish
in 1995-1996 and with a new hatchery in the Gorgan region is expected
to reach 20 million fingerlings (Abzeeyan, Tehran, 7(6):V,
1996). IRNA reported on 31 August 1998 that 24 million fry had
been raised since March of that year, a 15.3% increase over the
previous year and 20 million fry are now released each year. The
Shahid Rajaee Fish Aquaculture Center at Sari, Mazandaran produces 5.5
million sturgeon fingerlings annually, released in 13 Caspian Sea
rivers (IFRO Newsletter, 28:3, 2001). The only
species not produced is Huso huso and the most popular is Acipenser
persicus for its better quality caviar. The young are fed on
daphnia and later oligochaetes (white worms). Fingerlings may be grown
to 10-15 cm length before being released in the Safid River about 20
km from the sea to imprint on the river. In 1987 2.28 million
fingerlings were released and in 1993 6.5 million from the Beheshti
Hatchery. In 1993 a closed system fish culture plant was opened at
this hatchery to produce at least 5 million sturgeon fingerlings
annually (Abzeeyan, Tehran, 4(9):IV, 1993; see also Anonymous
(1993c)). A later report mentions culture of Huso huso in
addition to the sturgeon species mentioned above for the Dr. Beheshti
Sturgeon Hatchery, and production of fingerlings exceeded 60 million
in 1991, the best year from 1973 to 1993 (Abzeeyan, Tehran, 5(3
& 4):IX-X, 1994).
About half a million fingerlings were produced
in autumn 1995 in Mazandaran province (Iranian Fisheries Research
and Training Organization Newsletter, 9:6, 1995). In 1996, it was
expected that 15 million sturgeon fingerlings would be produced from
hatcheries, the main species being Acipenser persicus, Acipenser
stellatus and Huso huso. Fingerlings would be 3-5 g in
weight when released in the Safid River (Abzeeyan, Tehran,
7(2):IV, 1996). The Shaheed Beheshti Fish Propagation and Rearing
Complex of Shilat (Iranian Fisheries Company) produced 9 million
sturgeon fingerlings in 1997, each 3-5 g, for restocking (Bartley and
Rana, 1998b). Eggs are incubated for 5-7 days. Fingerlings are fed on
live Artemia, Daphnia and oligochaetes in 2.5 sq m
circular tanks from day 15 (60-80 mg) and then in earthen ponds for 50
days to the 2-3 g size. The fingerlings remain in the release river
for 10-15 days before entering the Caspian Sea.
The International Sturgeon Research Institute, which opened in 1994 near Rasht, released
22 million fry in 1996-1997 (Bartley and Rana, 1998b). The Institute
carries out varies research programmes, e.g. on the histology of the
gonads of reared sturgeons which have been found to be the same as
sturgeon in nature (Bahmani and Kazemi, 1998).
Abdolhay and Tahori (2006) give fingerling production for this species as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
81 |
74 |
65 |
0 |
31 |
| Injected broodstock |
29 |
24 |
19 |
0 |
10 |
| Spawning rate * (%) |
89.6 |
79 |
66 |
0 |
80 |
| Fertilisation rate (%) |
70 |
55.5 |
49 |
0 |
71 |
| Survival rate in incubators
(%) |
53 |
53.9 |
48 |
0 |
75.1 |
| Survival rate in tanks (%) |
80 |
70.1 |
68 |
0 |
79 |
| Stocking density in ponds
(fish/ha) |
88,333 |
74,580 |
63,752 |
0 |
65,000 |
| Survival rate in ponds (%) |
65 |
79 |
71 |
0 |
65.1 |
| Fingerling production (x
1000) |
1327 |
447 |
1816 |
0 |
617 |
* Rate of response to hormone injection
An experimental approach to conservation of stocks has been the raising of
sturgeon artificially to a size where they produce caviar. The Shahid Beheshti
sturgeon aquaculture centre raised a member of this species to 121 cm, 11.5 kg
and 8 years of age when it yielded 1.4 kg of caviar (Iranian Fisheries Research
Organization Newsletter, 37:2, 2003; Iranian Fisheries Research
Organization Newsletter, 40 & 41:4, 2004).
Shevchenko et al. (1999) summarise rearing technology for A. gueldenstaedtii in Iran. Fingerlings
are raised on artificial feeds in 1-4 cu m plastic tanks for up to 180
days. A mean mass of 120 g is attained, with a maximum of 300 g. Growth rate of
different age groups varied from 1.59 to 0.56% and daily weight gain was from
4.23 to 1.42%. The mean daily increment was affected by stocking density, daily
rations, oxygen content, feed quality and maintenance of feeding routine.
Falahatkar and Amini (2003) give further details on propagation from broodstocks
including maturity duration, oocyte diameter and weight, motility and density of
spermatozoids, time taken to reach 4 and 16 cell divisions, incubation duration,
fertilisation percentage achieved at each stage, mortality rate during
incubation, number of larvae obtained from each broodstock, number of larvae per
gramme, weight of each larva, and morphometric parameters and age for each broodstock.
Akrami et al. (2005) found cladocerans, copepods and chironomid larvae were secondary prey
items of fingerlings in one earthen pond with ostracods occasional prey, while
in another pond all these were secondary prey. Condition factor and growth
decreased as weight and length of fingerlings increased. Growth was was
negatively allometric (b<3).
De Meulenaer and Raymakers (1996) give figures for Iranian hatchery
production from 1983 to 1992 as 1.03 to 6.61 million fingerlings (mean
2.9 million) although mature adults are becoming increasingly
difficult to catch for stripping of eggs and sperm. These Iranian
hatcheries are much smaller than Russian ones which produced about 25
times this number on average annually from the Volga River hatcheries alone.
There is an extensive Russian literature on how to raise sturgeons,
e.g. Mil'shtein (1957; 1972), Marti (1972), Barannikova (1987) and
Dettlaff et al. (1993). A recent (1984-1986) estimate of this
species in the Caspian Sea is 47.7 million fish with 24-28% produced
by artificial means.
All sturgeons are particularly threatened on the spawning migration
when they concentrate in rivers (Rochard et al., 1990).
Sturgeons in the Aras River on the former Soviet-Iranian border, for
example, are threatened by dams and water diversion schemes (Zakharyan,
1972). However this is not an annual migration so the populations are
not subject to loss every year. The common problems encountered by all
Caspian sturgeons are dams and weirs which block reproductive
migrations of adults upriver and also of young and adults returning to
the sea, water abstraction for irrigation which reduces flow or even
dries up a river, degradation of the river bed by extraction of gravel
for construction or the change in silt deposits by the filtering
effects of dams, increased water clarity enables predators to be more
effective changes in the oxygen and temperature regimes caused by
water abstraction, retention of water behind dams or untimely release
from dams, pollution, attraction of adults into irrigation channels by
their strong water flow and changes in the invertebrate fauna on which
the young feed in rivers (Vladykov, 1964; Anonymous, 1970c; Whitney,
1979; Rochard et al., 1990). Variations in Caspian Sea levels
also had effects (Khodorevskaya et al. 1997). For Huso huso
these include lowered accessibility to feeding sites and variations in
food abundance which lead to decreases in relative weight gain and to
a halving in the number of females. The growth and survival of
juvenile Acipenser gueldenstaedtii in the Volga River delta
during their first winter is affected by lower water levels.
Stocks in their sea life were fairly safe until trawling was
introduced. There are restrictions on trawling in the sea to reduce
loss of young sturgeons (Ricker, 1970) and trawling is banned in the
territorial waters of Azerbaijan (Markarova and Alekperov, 1989). It
has been suggested that the Caspian Sea level should be maintained at
-28.5 m or above to retain water productivity on which sturgeons
ultimately rely. A 1 m decline in level can reduce fish food supply by
60% and hinders migration to feeding grounds, another 20% loss (Petr, 1987).
The institution of closed seasons for fishing and restrictions on
techniques used to limit juvenile catches have been implemented in the
former U.S.S.R. The fine for illegal possession of a Huso huso
was about £280 in 1977. Fish lifts have been built on the Volga River
about 5000 km upstream from the Caspian Sea to transport sturgeon
around the Volgograd Dam. The system transports about 10-20% of
migrating Huso huso, Acipenser gueldenstaedtii and A.
stellatus but is relatively inefficient (Rochard et al.,
1990). The poor situation is compounded by the lack of suitable
spawning conditions above the dam and by adults having to migrate
downstream through the dam's turbines. The turbines have wide blades
and rotate slowly so most adults cannot make it through although the
young are short enough to survive the transit. Khodorevskaya et al.
(1997) summarise the decline in catches of this species after the
regulation of the Volga River flow by the Volgograd Dam, built in
1958-1960, which cut off as much as 80% of the spawning grounds.
In Iran baiting hooks with oilcloth or fish was banned in 1952 as
this method took large numbers of immature sturgeon (Vladykov, 1964).
Some problems however may be intractable such as local consumption of
immature fish rather than release or registration in catches. This
lack of registration prevents adequate assessment of the catch and
effective management suffers (Vladykov, 1964). Iran has recently taken
a number of steps to protect the caviar resources including a
reduction in the annual catch from 3000 tons (sic, probably
tonnes) to 1500 tons, restricting export to the government rather than
private companies, combating the illegal caviar trade, and the setting
of export quotas and price controls for Caspian Sea countries (Abzeeyan,
Tehran, 4(5):VI; 4(7):VI, 1993). Gill netting was prohibited in 1995 (Abzeeyan,
Tehran, 6(5, 6):IV-V, 1995). The break-up of the Soviet Union led to
smuggling and overfishing in the newly independent countries around
the Caspian but Iran was able to stabilize world prices by reducing
its caviar exports by 30%. Until 1992 Russian caviar dominated the
world market but more recently Iran became the main supplier with
income for 1989-1994 twice that of 1979 and 1989 (Abzeeyan,
Tehran 5(1 & 2):VII, 1994; Ferguson, 1994). Nevertheless, some
authorities believe overfishing by the five Caspian nations,
particularly in the sea where immature fish are taken along with
adults, will result in the extinction of the sturgeon species there (Los
Angeles Times, Part A, page 1, 28 August 1993). An account of the
caviar black market in Dagestan is given by Chenciner (1998).
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 2% for this species among sturgeons captured.
Lelek (1987) and Maitland (1991) report this species as
"vulnerable" in Europe because it grows and matures slowly,
it is exploited, affected by pollution and killed by river
engineering. Critically endangered in Turkey (Fricke et al., 2007). This species showed the greatest decline among Iranian
sturgeon species through overfishing of younger age groups and habitat
alterations (RaLonde and Walczak, 1970b). Kiabi et al. (1999)
consider this species to be vulnerable in the south Caspian Sea basin
according to IUCN criteria. Criteria include commercial fishing,
medium numbers, habitat destruction, medium range (25-75% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin. IUCN ranks all stocks as endangered (Vecsei, 2001).
Further work
The main concern with all sturgeon species is maintaining a viable
commercial stock. Poaching has caused a decline in the available
number of fish which can be used for breeding and moreover more than
30% of breeders do not respond to hormone stimulation (Kokoza et al.,
1995). There were 6 times more nets in Azerbaijan waters and 4 times
more in the Volga River delta in 1993 than in the 1980s. The legal
catch will probably have to be completely prohibited (Ivanov et al.,
1995). Efron (1993), for example, describes the "caviar
crisis" in the Caspian Sea but problems have long been evident
(Anonymous, 1961a). In 1996, 1 t of caviar was seized from smugglers
in Gilan and one smuggler was fined 20 billion rials (IRNA, 28
July 1997, www.netiran.com). Maintenance of the stock may only be
possible by hatchery production as river regeneration is no longer
feasible because of dams. Mortality in Iran for hatchery reared eggs
of 2 months age was 30-35%, for larvae 20-40%, and for fingerlings
30-40%, a satisfactory level but this could always be improved on (Petr,
1987). Yearly production of sturgeon fingerlings in government
hatcheries in Iran was 1.03 millions in 1983, 1.11 in 1984, 1.13 in
1985, 2.28 in 1986, 3.10 in 1987, 3.16 in 1988, 3.15 in 1989, 4.34 in
1990, 6.60 in 1991, and 3.20 in 1992 (Emadi, 1993a). The 1996 hatchery
production of sturgeon was 12.5 million in 1996 (Bartley and Rana,
1998a). A hatchery facility in Gilan covers 136 ha, produces up to 7
million sturgeon fingerlings a year with plans for up to 20 millions,
and is said to be the largest and most modern sturgeon hatchery in the world.
The Israelis farm osetra and caviar from this species was on sale at
Philadelphia airport at US$75/oz on 19 April 2006.
A detailed comparative study of the morphology of this species and Acipenser
persicus in Iran would enable the young and adults to be clearly
distinguished as well as stocks within each species as a management tool.
Sources
See under the family account.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Acipenser nudiventris
Lovetzky, 1828
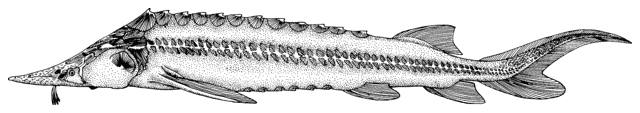
Common names
شيپ (= ship, šep or sheap),
تاس ماهي (= tas mahi, included under this name with A.
gueldenstaedtii and A. persicus when eggs are large for
fisheries statistics), tass mahi shekam brahne, سگ ماهي
(sag mahi), ماهي خاويار (= mahi-ye kaviar,
meaning caviar fish), keshdi, shenavar.
[kalamo, kelemo or kulamo, xazar kalamosu, gaya baligi, girt, ag-gyal or
bich-nyarya in Azerbaijanian; sip or bekre balyk in Turkmenian; spiny sturgeon, thorn sturgeon, fringebarbel sturgeon,
barbel sturgeon,
bastard sturgeon].
Systematics
Acipenser nudiventris was originally described from the Aral Sea.
Acipenser schypa Eichwald, 1831 is a synonym. It is credited
to Linnaeus by Eichwald but not described by Linnaeus; if this name is
available then it is preoccupied by Acipenser schypa
Gueldenstaedt, 1772 (Eschmeyer et al., 1996). Note that Holčík (1989) gives the
spelling as shypa. Acipenser shipa
Lovetsky, 1834 and Acipenser schypa Kessler, 1856 are synonyms.
Acipenser schip Eichwald, 1841 is presumably a misspelling. Acipenser
shyp Forster, 1767 may have priority but this has not been
investigated.
Acipenser nudiventris derjavini Borzenko, 1950 was described
as the Caspian Sea subspecies, as the type locality for the nominate
subspecies is the Aral Sea, but derjavini is no longer recognised (Holčík, 1989).
A hybrid with Acipenser stellatus is reported from the Safid
River (Nedoshivin and Iljin, 1927) and it also hybridises with Huso
huso (Berg, 1948-1949).
V. D. Vladykov points out (in litt., 1973) that ship (in Russian) is probably a Turko-Tartar word referring to a hybrid
since this species has a snout intermediate in length between that of Acipenser
gueldenstaedtii, which is short, and that of Acipenser
stellatus, which is long. The Russian word means prickle or thorn and has given rise to the common names for this
fish in English of "spiny" or "thorn" sturgeon. Acipenser
nudiventris, as its name indicates, has weakly developed or worn
ventral scutes so the names spiny or thorn sturgeon are inappropriate.
Vladykov recommends "sheap" as the common name to avoid
confusion with the word "ship" in Russian (or for that matter in English).
Nucleotide diversity is much lower than other sturgeons in
the Caspian Sea, possibly due to a smaller population size. Haplotypes of
sturgeons from the Ural River in the north Caspian and Iranian waters were
significantly different (Qasemi et al., 2006).
Microsatellite studies indicate that there is more than one population in the
south Caspian Sea and these populations are different from the Ural River one in
the north Caspian Sea (Safari et al., 2007; 2008; 2008).
Key characters
This species has a continuous and thick lower lip, usually more
than 50 scutes laterally, fimbriate barbels, and a transverse mouth.
Morphology
The body is deepest at the first dorsal scute. The rostrum is rounded and
conical in shape in adults, more spatulate in young. Adults are covered with
minute scutes giving a sandpaper texture although visually appearing
smooth. Dorsal fin rays 39-57 and anal fin rays 23-37. Dorsal scutes 11-17,
lateral scutes 49-74 and ventral scutes 10-17. There are no large plates on
the body between the scutes. Scutes lack a hook and even juveniles have this
usual feature barely developed. Ventral scutes are lost or absorbed in large
adults (hence the scientific name). Gill rakers 24-45. Chromosome number is
2n=116 ± 4, 2n=118 ± 2,2n=118 ±
3 (Klinkhardt et al., 1995; Nowruzfashkhami et al., 2000; Havelka
et al., 2011). Nourouz Fashkhami et al. (2009) gives details of a method to produce the
most metaphase plates.
Sexual dimorphism
Females are larger than males. Abdurakhmanov (1962) reports a
greater average number of gill rakers in females, a longer postorbital
distance in females, and longer caudal peduncle, pectoral fin, pelvic
fin, snout, eye and snout tip to barbel distance in males.
Colour
The back is olive-green, grey-green or grey-blue, fading to a yellowish-white belly. Fins are
grey.Juveniles mayhave the same colouration as adults or be almost black
dorsally and laterally with a white belly.
Size
Attains 2.21 m and 127 kg. Safid River fish reached 43 kg, weighed
when frozen, with the average being 20.1 kg, in 1914-1915 (Nedoshivin and Iljin, 1927).
Distribution
Found in the Black, Caspian and Aral seas and their drainages but extinct in
the latter. In the Caspian Sea it is most common in the south, being rare in the
Volga River for example. A long residency in fresh water probably
accounts for their scarcity since mortality from winter and predators
is high. Migrations in the Kura River extended 650 km and in the Aras
River 300 km until the Mingechaur Dam was built. Enters the Aras, Astara, Safid, Tajen and Babol rivers in Iran (Derzhavin, 1934; Armantrout, 1980;
CITES website, downloaded 5 April 2004).
Also reported from Hasan Kiadeh by Derzhavin (1934) and by V. D.
Vladykov based on field work notes made in 1962. Rostami (1961) also records
this species from several localities on the Safid River. More recent works
only report it from the Safid River, the southeast Caspian Sea,
southwest Caspian Sea and south-central Caspian Sea (Kiabi et al., 1999;
Abdoli and Naderi, 2009)
and from the Safid River (Abbasi et al., 1999). Vecsei et al.
(2002) consider it as rarely observed in Iran,
Zoogeography
Presumably a relict of the past isolation of the Aral-Caspian-Black
seas from the Mediterranean-Atlantic. This species is reported from
the Karakum Canal and Kopetdag Reservoir in Turkmenistan by Shakirova
and Sukhanova (1994) and Sal'nikov (1995) and may eventually reach the
Tedzhen (= Hari) River basin of Iran.
Habitat
A rare species in trawl catches but known from feeding grounds
along the eastern coast of the south Caspian Sea (Legeza, 1973). Only
100 fish enter the Kura and the Ural stock, an undammed river, is in the low
thousands (Vecsei et al., 2002). This species was never as abundant as
other sturgeons because young spent 2-8 years in fresh water where predators
abound and food is more limited (Vecsei et al., 2002) As an adult,
it favours the areas near river mouths with muddy bottoms. Markarova et al. (1991)
state that its main abundance is south of the mouth of the Kura River
and that it ascends the Safid Rud to spawn, although in smaller
numbers than the Kura River. This species is uncommon in Iranian
waters, only 2.5% in numbers and 4% in weight of the Safid River catch
in 1914-1915 (Nedoshivin and Iljin, 1927; RaLonde, 1970b), and catches
in Azerbaijan are not more than 5% of all sturgeons (Markarova and
Alekperov, 1989). It is usually found over mud near shore at 30-60 m.
Age and growth
Maturity is attained 6-13 years in males and begins at 12-22 years
in females and most are mature at 14 years. Females grow faster than
males. Caspian fish grow faster and larger than those in the Aral Sea.
The oldest fish in the Kura River was 35 years (Markarova et al.,
1991), and maximum age is 36 years. Growth is rapid with one-year-olds
in the Caspian being 23-29 cm long and weighing 40-60 g.
Food
Markarova et al. (1991) found sheap in the south Caspian Sea
to eat fishes such as Atherina, Neogobius (presumably including
related genera), Benthophilus
and Clupeonella, polychaete worms (Nereis), and various
crustaceans. Molluscs play a small part in their diet but eggs of
other sturgeons and the crab Rhithropanopeus harrisii are very
important. The crab, an accidental introduction to the Caspian Sea at
the end of the 1950s, comprises 70% by weight of the food taken. Young
sheap in the Kura River feed on insect larvae such as caddisflies,
dragonflies, mayflies and stoneflies. Hashemyan et al. (2005) found diet
in A. persicus, A. stellatus and A. nudiventris in coastal
waters of Mazandaran and Golestan at depths less than 20 m to consist of
annelids (50.8%), amphipods (41.5%), small fish 4.8%), decapods (2%) and
bivalves (0.9%). Fish shorter than 40 cm fed mostly on shrimps, polychaetes and
gammarids, 41-80 cm fish fed on shrimps, gammarids, polychaetes, bivalves and
smaller fish, while fish greater than 80 cm fed mostly on shrimps and smaller
fish.
Reproduction
A spawning migration to rivers occurs year-round but peaks in
March-April and in October-December in the Kura River of Azerbaijan (Markarova
et al., 1991). The spring run begins at 6.2-13.0°C
while the fall run is at 12.0-17.9°C.
Males predominate over females by 3-6 times. Spawning occurs in
April-May at water temperatures of 10-25°C
and normal development occurs between 11.0 and 17.1°C.
Eggs are laid on pebbly substrates at current speeds of 1-2 m/sec.
Fecundity in sea-caught fish was up to 959,100 eggs (Markarova et
al., 1991). Elsewhere egg numbers may reach 1,290,000 with
diameters up to 3 mm. Fry soon migrate to the sea. Spawning by
individuals is not an annual event but occurs at intervals of 2-3
years for females and 1-2 years for males, allowing for recovery and fattening. Some spent fish may remain
in the Kura River for up to 8 years.
Halajian et al. (2007) used biopsies to determine sex and sexual maturity
stages in 5 and 6 year old fish. Males matured sooner than females. Shalouei and
Imanpour (2009) found that spermatozoa were immotile in ovarian fluid because of
the high concentration of potassium and osmotic pressure.
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Mokhayer and Anwar (1973) report on parasites of sturgeons including
this species (see under Acipenser gueldenstaedtii). Mokhayer
(1976b) reports gas bubble disease in Iranian sturgeons without
specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Sattari et al. (2002) record Cucullanus sphaerocephalus,
Eustrongylides excisus, Skrjabinopsolus semiarmatus,
Leptorhynchoides plagicephalus and Eubothrium acipenserinum, the
fauna being similar to other sturgeons because of their piscivorous feeding.
Sattari and Mokhayer (2005a; 2005b) recorded the occurrence of parasites in this
species from the Iranian southwestern and central coast of the Caspian Sea. The
species found were the nematodes Cucullanus sphaerocephalus and
Eustrongyloides excisus, the cestode Eubothrium acipenserinum, the
acanthocephalan Leptorhynchoides plagicephalus, and the digenean
trematode Skrjabinopsolus semiarmatus. General conclusions were that the
diversity of parasites was less in Iranian waters than in the northern Caspian
Sea, perhaps a reflection of the more varied habitat, its productivity and the
carbonate ions differing between the two regions. The diversity of parasite
seems to have declined over time also, perhaps as a result of unfavourable
environmental conditions, particularly in the freshwater ecosystem which limits
the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Trichodina sp.
Economic importance
The relative scarcity of this species accounts for it being not
more than 1% of the Caspian Sea catch of sturgeons. The highest catch
in the Kura River seems to have been 6000 fish in the 1930s. The Iranian catch
after the CITES website (downloaded 5 April 2004) was:-
| Year |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
| Tonnes |
1.9 |
22.4 |
19.0 |
17.5 |
17.3 |
15.7 |
16.6 |
13.5 |
19.4 |
21.0 |
3.5 (spring only) |
Moghim (2004b) records the total Iranian catch as 2% of the total sturgeon
composition and it is declining. In 1972 the catch per unit effort was 67 tonnes
and 0.5 kg/boat/day but by 2002 it was 15 t and0.09 kg/boat/day.
Conservation
Reduction in flow of the Kura River, the main spawning ground, is
four times less than before regulation (5.5 km3/year
compared to 20-24 km3/year). Sheap find it difficult to
enter the river. Artificial propagation will be the only way to
maintain the population. Between 2.9 and 6.2 million young sturgeon
were released annually in the Caspian Sea from 1966 to 1971. This
situation is mirrored in Iranian rivers such as the Safid Rud. This
species is also particularly sensitive to oil pollution when young.
There are reports that all but the Ural River population are on the
verge of extinction (The Sturgeon Quarterly, 2(2):1, 1994; Vecsei, 2001). It
is already extinct in the Aral Sea (DeSalle and Birstein, 1996). This
species is protected in Iran since populations along the southern
Caspian shore have been greatly reduced and there are not enough mature fish for
fish farming (Bartley and Rana, 1998b; Vecsei et al., 2002).
However the CITES website (downloaded 5 April 2004, but citing September 2000 data) reports
that Iranian hatcheries still obtain some breeders from rivers. CITES also notes
that the number of fishing stations for this species in Iran has been decreased
by half, use of gillnets for Rutilus spp. prohibited as they take
sturgeons too, egg removal by caesarian section instituted, release of fry from
a breeding stock of 3000 fish, and lower export quotas instituted.
Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
15 |
38 |
16 |
50 |
25 |
| Injected broodstock |
14 |
21 |
29 |
32 |
19 |
| Spawning rate * (%) |
86 |
95.2 |
78 |
74 |
75 |
| Fertilisation rate (%) |
80 |
71.5 |
73 |
70 |
72 |
| Survival rate in incubators
(%) |
54 |
61.5 |
51 |
49 |
74 |
| Survival rate in tanks (%) |
70 |
74.7 |
76 |
61 |
66 |
| Stocking density in ponds
(fish/ha) |
92,100 |
77,005 |
56,194 |
87,986 |
61,667 |
| Survival rate in ponds (%) |
71 |
60 |
85 |
34 |
20 |
| Fingerling production (x
1000) |
1143 |
1782 |
1819 |
1414 |
1311 |
* Rate of response to hormone injection
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 1% for this species among sturgeons captured.
This species is sensitive to pesticides such as diazinon. The LC50
(96 h) was 4.6 mg/l and lowered erythrocyte and lymphocyte counts were recorded
with a significant increase in neutrophil counts (Khoshbavar Rostami and Soltani,
2005). Parand Avar et al. (2008) studied the effects of photoperiod
during feeding by juveniles on Daphnia. Uptake was higher in dark
conditions. Shalouei et al. (2009) studied extenders of spermatozoa
motility and Shalouei et al. (2008) the correlation between seminal
plasma indices and spermatozoa motility..
Maitland (1991) lists this species as "endangered" in
Europe because of the declining population, slowness in growth and
maturity, exploitation, and pollution and dams on the spawning
migration. Birstein (1993) and the CITES website (downloaded
5 April 2004) also consider it be endangered. Critically endangered in Turkey
(Fricke et al., 2007). Robins et
al. (1991) list this species as important to North Americans.
Importance is based on its use in aquaculture and as food.
Kiabi et al. (1999) and Moghim (2004b) consider this species to be critically
endangered in the south Caspian Sea basin according to IUCN criteria while the
IUCN gives endangered (Vecsei et al. (2002).
Criteria include commercial fishing, few in numbers, habitat
destruction, limited range (less than 25% of water bodies), absent in
other water bodies in Iran, and present outside the Caspian Sea basin.
See also under A. gueldenstaedtii.
Further work
See under A. gueldenstaedtii.
Sources
See under the family account.
Iranian material: None.
Comparative material: BM(NH) 1879.11.14:56, 1,
255.0 mm total length, U.S.S.R., Tschinas (no other locality data); BM(NH)
1879.11.14:57, 1, 217.0 mm total length, U.S.S.R., Tschinas (no other locality
data); BM(NH) 1897.1.25:9, 1, 411.7 mm total length, Romania, Orsova, lower
Danube (no other locality data).
Acipenser persicus
Borodin, 1897
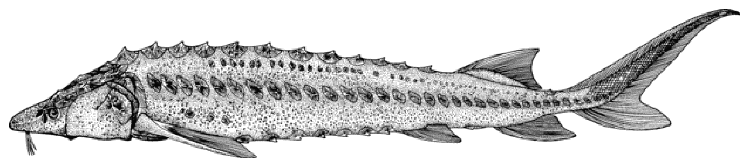
Common name
قره برون (= qara burun, kara burun, kareh burun or ghareburun, meaning black nose),
تاس ماهي
(= tas mahi, this term includes A. gueldenstaedtii), دراكول (= darakul),
تيريج (= tirij),
تاس ماهي ايراني
(= tasmahi-ye Iran), تاس ماهي ايراني
(= tasmahi Irani or tasmahi-e-Iran), سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), cetra.
[nara, nyarya or njara, neresi, Kur narasi for natio kurensis, or bekra in
Azerbaijan; perseya, gunorta perseya, bekre balygy in Turkmenian; kurinskii or persidskii osetr, i.e. Kura or Persian
sturgeon in Russian].
Systematics
The type locality of this species is the Ural and Kura rivers.
Regarded as not distinct from or a subspecies of Acipenser gueldenstaedtii by
some authors (see Borodin, 1926; Berg, 1948-1949; Whitehead et al.,
1984-1986; Keyvanfar et al., 1987; Keyvanfar, 1988; Ruban et al.,
2008, 2011) but Luk'yanenko et al. (1974), Artyukhin and Zarkua (1986), Vlasenko, Pavlov and Vasil'ev in
Holčík (1989), Keyvanfar and Nasrichari (1999), Pourkazemi et al. (2000), Subbotkin and
Subbotkina (2001), Ghorbani and Hajimoradloo (2002), and Gharei et al. (2005) restore it to a full
species on meristic, morphological, ecological, caviar proteins, serum proteins, mtDNA, genomic DNA and
immunological grounds. And again, Birstein et al. (2005) consider it not
to be distinct from Acipenser gueldenstaedtii on the basis of molecular
analyses. Ruban et al. (2008, 2011) used meristic, morphometric and
molecular data to come to the same conclusion. Acipenser gueldenstaedtii has a complex intraspecies structure
according to Birstein et al. (2005) and, depending on the rivers and
populations sampled for any given studies, conflicting results can arise. For
the moment, the taxon A. persicus is retained here as distinct until
further resolution of the problem is attained, although given the decimation of
populations this may not be possible.
Moghim et al. (2009) report at least 18 groups that segregate
spatially and temporally for spawning in the Caspian Sea basin. Khoshkholgh
et al. (2011) using mitochondrial DNA found the that the Safid River
population was reproductively isolated. Differences between other populations (Astara,
Nowshahr, Bandar-e Torkoman) were not significant and most variation occurred
within populations. Electrophoretic studies of blood proteins coupled with morphological
data indicate that Gorgan and Safid River populations are two
geographical races (Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 42, 1996).
Chakmehdouz Ghasemi et al. (2010, 2011) using microsatellite markers found no
differences between Safid and Gorgan River populations for average number of
alleles per locus and for observed heterozygosities although genetic difference
was significant, warranting separate restocking efforts and conservation of gene
pools.
The type subspecies is found in the Caspian Sea
and Acipenser persicus colchicus Marti, 1940 in the eastern
Black Sea. A natio kurensis Belyaeff, 1932 is reported from the
Kur River of Azerbaijan within Acipenser gueldenstaedtii persicus.
Hybrids with Acipenser gueldenstaedtii are reported from the
Volga and the Caspian Sea and have been produced artificially (Vasil'eva et al., 2001).
Two syntypes of Acipenser persicus are possibly in the
Zoological Institute, St. Petersburg (ZISP, formerly ZIL) (Eschmeyer et al., 1996).
Key characters
This species has long been confused with A. gueldenstaedtii,
but can be distinguished by a more elongate, massive and downward
curved snout, a white belly and a grey-blue back. A drawing in Vlasenko, Pavlov and Vasil'ev in Holčík
(1989) of the two species has a snout length in head length of 3.2 as
opposed to 4.3 for Acipenser gueldenstaedtii but figures of
snout length in total length overlap for the two species. The
interorbital distance is much less in the Persian sturgeon (29.2-30.5%
of head length) than in A. gueldenstaedtii (Artyukhin and Zarkua, 1986)
but in small specimens examined by me from Iran, some
had gueldenstaedtii interorbital distance and persicus snout length.
Morphology
The Persian sturgeon is slender with an elongate and cylindrical
body, a long head, and a narrow, medium length (5.6% of total length),
massive and usually depressed snout. The snout width near the mouth is
37% of head length. The pectoral fins are relatively small and have
only a weak bony ray. There are usually 1-4 rows of smaller,
longitudinally arranged, bony plaques between the scutes of the dorsal
and lateral rows and sometimes between the lateral and abdominal rows.
The barbels are located relatively closer to the snout tip than those
of A. gueldenstaedtii.
Sheibani and Adib Moradi (2000) described the histology of the pylorus and
pyloric caecum in this species and Sheibani and Pahlavan (2003) the
developmental histology of the liver and pancreas from fry to fingerling.
Dorsal fin rays 27-51, anal fin rays 16-35 according to Holčík (1989)
and 30-49 and 18-32 according to Berg (1948-1949) for Kura
River fish. Gill rakers 15-36. Dorsal scutes 7-19, lateral scutes
23-50 and ventral scutes 7-13 according to Holčík
(1989) while Berg (1948-1949) gives 5-13, 21-42, and 7-14 respectively
for scutes from Kura River fish. Safid River fish have a higher number
of lateral scutes than fish from the Kura River.
The chromosome number is 2n>200 (Nowruz Fashkhami, 1996), later amended to
2n=258 ± 4 (Nowruzfashkhami et al., 2000; Havelka et al., 2011).
Sexual dimorphism
Females are heavier and longer than males of the same age.
Colour
The back is greyish-blue to dark blue, the flanks with a steel-blue
sheen, the head is lighter than the back, and scutes are lighter in colour
than the background, usually pale yellow in adults but copper-gold in
young. The belly is off-white, sometimes slightly yellowish.
Size
Reaches 2.42 m, possibly 2.50 m, and 76 kg, possibly 80 kg
(Machacek (1983-2012), downloaded 27 July 2012). A specimen caught by
the Bandar-e Torkeman fishery weighed 63 kg, as opposed to the usual
weight of 18-20 kg (Abzeeyan, Tehran, 5(3 & 4):V-VI, 1994). Males
migrating into the Volga River typically weigh 20-30 kg and females 30-35 kg (Vecsei
and Artyukhin, 2001).
Distribution
Found in the Caspian Sea, migrating to the north but mainly in the
south in the Kura River of Azerbaijan and rivers of Iran where it is more common
than A. gueldenstaedtii (Ivanov and Katunin, 2001). Also in the
eastern Black Sea as a distinct subspecies.
In Iran, it is found from the Astara River in the west to the Gorgan
River in the east (Armantrout, 1980), but apparently not the Atrak River on the
border with Turkmenistan (Berg, 1936). Distribution includes the Safid River (to
Kisom and "Musachayu"), Shalman,
Golchan, Langerud, "Djef", "Youssefabad",
"Tchontchenan", Dehkah, "Polrud", Sorkhrud, Feridounkenar,
Talar, Tajan, Neka, "Palarud", Babol, "Mirerud", and "Ferikhabad"
(Kozhin, 1957; Rostami, 1961; Armantrout, 1980). Also
reported from Kargan, Kopurchal, Golshan, Larim, Nirroud, Tazeabad, 12 Bahman, Nevissi, Iz
Deh, and Hasan Kiadeh by V. D. Vladykov based on field
work notes made in 1962. Reported more recently as occurring in the Gorgan,
Babol and Aras rivers by Holčík (1989), in the Gorgan, Gharasu, Tajan, Babol, Haraz, and Safid rivers,
Gorgan Bay, the southeast Caspian Sea,
southwest Caspian Sea and south-central Caspian Sea by Kiabi et al.
(1999) and Abdoli and Naderi (2009) and in the Safid River and Anzali Talab by Abbasi et al. (1999).
It used to ascend the Aras River but numbers in Iranian reaches were always
small. Some literature
records of A. gueldenstaedtii may be this species.
Zoogeography
Presumably a relict of the past isolation of the Black-Caspian seas
from the Mediterranean-Atlantic.
Habitat
This species predominately inhabits the southern part of the
Caspian Sea but does not form dense concentrations. Catches do not
exceed 10-20 fish in 30 minutes of trawling. In winter to spring it is
concentrated in the eastern coastal region and moves north in summer.
In spring, maturing fish are concentrated in the southwest (Legeza,
1973). There is no seasonal variation in depth distribution in the
south Caspian Sea in contrast to the middle Caspian. It is found on
silty bottoms in the south Caspian Sea, sometimes with a sand
admixture, at a temperature range of 4.1-28.0°C
and a salinity range in the sea of 8.59-14.2‰. This species is more
stenohaline than the others, preferring waters with higher salinity as
in typical marine Caspian water and is also more sensitive to lowered
oxygen levels (Legeza, 1972). Kazemi et al. (2003) found that osmoregulatory
ability and development of chloride cells increased during growth, enabling the
fish to transition between fresh and more saline waters. Khodabandeh et al.
(2007) found fry transferred from fresh water to 7.5 and 10‰ sea water
experienced 100% mortality after one hour acclimation; cortisol treatment
increased the ability of fry to withstand these salinities. Ivanov and Katunin (2001) in a trawl survey along
the Iranian coast found 14.2 fish/trawl in the west and 6.7 fish/trawl in the
east with undersized and juvenile fish in the west at 57 fish/trawl. The higher
western catches were attributed to the presence of more rivers, in particular
the Safid River. The general abundance of this species was 8.775 million fish.
This species prefers fast rivers for spawning and migrate long
distances. In the Volga River they migrate at an average speed of 22.6
km/day. They may remain in fresh water after spawning for a year or
more although most return to the sea. These freshwater fish overwinter
in deeper holes and feed intensively on fishes, crustaceans and
molluscs. Larvae move downstream immediately after hatching. Cultured fingerings
can be released safely and optimally into the rivers and estuaries of Iran at an
age of 33-35 days after yolk-sac absorption at a weight of 1.8-2.4 g and 6.2-7.5
cm length (I.F.R.O. Newsletter, 30-31:5, 2002).
Bahmani et al. (2001) have shown that broodfish caught by seines in
the Safid River were less stressed than fish caught by gillnets in estuaries.
Age and growth
Maximum age for accidental catches in the Caspian Sea off
Azerbaijan is 32 years but most (82%) are 14-23 years old. Maturity is
attained between 8 and 13 years in the Kura River (Markarova and
Alekperov, 1989). Most fish entering the Kura River to spawn are 7-34
years old and the main spawning population is 11-24 years. Mean
lengths for Safid River fish are 161 cm for females and 141 cm for
males. Females have a faster growth rate than males. Growth rate is
faster than for A. gueldenstaedtii and in the Volga size and
weight is considerably higher. The numbers of this species and A.
gueldenstaedtii in the southern Caspian are about equal. Maximum
life span is 48 years.
Studies in 2007, however, when 50 stations were sampled in waters less than 10 m
deep, found this species to comprise 82.7% of the absolute frequency and 59% of
the biomass of the total sturgeon catch. A. gueldenstaedtii was last with
5.5% and 2.3% respectively (Iranian
Fisheries Research Organization Newsletter, 51:2, 2007).
Von Bertalanffy growth parameters in Iranian females are L
∞ = 225 cm and K = 0.066 or 207 cm and 0.079 and for males 197 cm and
0.084 or 186 cm and 0.105 depending on the methodology used. Total
mortality (Z) was 0.24-0.57 for females and 0.40-1.1 for males,
natural mortality (M) was 0.04 for females and 0.06 for males, fishing
mortality (F) was 0.47 for females and 0.34 for males, and optimum
fishing mortality was (F) 0.16 for females and 0.34 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
A sample of 31 males and 49 females from the Turkman Sturgeon Fishery Station in
2001 showed sexual maturity at more than 19 years for females and more than 17
years for males (Alavi et al., 2005). Fish taken at 9 fishing stations
along the Iranian coast numbering 4689 individuals had a mean length of 139.1 cm
for males and 153.4 cm for females, weights 19.95 kg and 29.09 kg respectively
and an age of 14.15 years and 16.59 years respectively. The sex ratio was 1:2.2
in favour of females and the majority of females (89.6%) were at level IV
maturity. An increase in sexual maturity of females occurred in autumn while
males were most mature from June to September (Falahatkar, 2006). Samples taken from the whole Caspian shore of Iran from 2002 to 2004 numbering
11,480 fish had a length range of 90-240 cm and growth parameters L∞
= 230 cm and K = 0.058 year-1 (www.shilat.com, downloaded 28
February 2007).
Bakhshalizadeh et al. (2011) collected data from commercial Iranian
fisheries (2008-2010) and found a maximum age of 39 years, growth parameters for
females were L
∞ = 173.07 cm, K = 0.1 year-1 and Z = 0.45 year-1
and for males 164.33 cm, 0.08 year-1 and 0.76 year-1.
Differences between this and previous studies were attributed to increased
fishing and environmental degradation.
Food
Diet is composed of molluscs, crustaceans including the introduced
crab (Rhithropanopeus harrisii), worms, chironomids and fish
such as gobies (Gobiidae) and small herrings (Clupeonella spp.).
Fish are a large part of the food of young sturgeon at sea. Azari
Takami et al. (1980) found adults to prefer fish, mostly
gobies, followed by crustaceans and two clam species Abra ovata
and Cerastoderma umbonatum in Iran. The zebra mussel is also
eaten as evidenced by a mass of these small clams from the stomach of
a 1.6 m, 35 kg female from Nevissi caught on 29 September 1962.
Reportedly the food diversity of this species is much less than for Huso
huso and Acipenser gueldenstaedtii.
Commercial sized fish feed particularly in the northern Caspian Sea (Ivanov and Katunin, 2001).
Hashemyan et al. (2005) found diet in A. persicus, A. stellatus
and A. nudiventris in coastal waters of Mazandaran and Golestan at depths
less than 20 m to consist of annelids (50.8%), amphipods (41.5%), small fish
4.8%), decapods (2%) and bivalves (0.9%). Fish shorter than 40 cm fed mostly on
shrimps, polychaetes and gammarids, 41-80 cm fish fed on shrimps, gammarids,
polychaetes, bivalves and smaller fish, while fish greater than 80 cm fed mostly
on shrimps and smaller fish. Immature A. persicus, less than two years
old, from fishing stations off Gilan fed on the benthic invertebrates, namely
the polychaetes Hypania sp., Hypaniola sp. and Nereis sp.,
the cumaceans Pterocuma sp. and Stenocuma sp., the clam Abra
ovata, and the crustaceans Paramysis sp. and Gammarus sp.
Adults fed mostly on fish (gobies, smelts and herrings). Haddadi Moghadam et
al. (2009) studied diet in fish collected in summer and winter in the south
Caspian Sea from 2004 to 2006. Food items were fishes (Neogobius sp.,
Atherina caspia, Clupeonella cultriventris (= caspia) and
invertebrates (polychaete worms such as Ampharetidae and Nereis diversicolor;
crustaceans such as Gammarus and Paramysis; and the bivalve
mollusc Abra ovata). The diet varied with season and size group and was
similar to A. stellatus. Imanpour Namin et al. (2010) studied
southern Caspian Sea fish and found the diet included Gobiidae, Mysidae,
Gammaridae, Nereidae, Ampharetidae, Pseudocumidae, Clupeidae, Syngnathidae,
Scorbicularidae, Cardidae and insects. Diet diversity decreased with increase in
fish size, from 8 to 5 to 2 items in length classes <30 cm, 30-60 cm and >60 cm.
Gobiidae dominated in the largest length class and Gobiidae and Mysidae in the
middle length class.
The account under A. gueldenstaedtii above gives
some comparative details of diet.
Reproduction
This species was long confused with the chalbash, A.
gueldenstaedtii, and was thought to be a late spring or early
summer spawning population of that species. The spawning run follows that of A.
gueldensatedti. Spawning runs are
dominated by the spring form and winter fish are very rare (Artyukhin
and Zarkua, 1986). Fecundity off Azerbaijan is up to 558,900 eggs (Markarova
and Alekperov, 1989) but may reach 840,000 eggs. In the Safid River it
attains 375,000 eggs. The eggs are brownish-grey and measure up to 3.8 mm in diameter.
The unusually large specimen caught by the Bandar-e Torkeman
fishery gave 22 kg of caviar, almost 35% of the body weight (Abzeeyan,
Tehran, 5(3 & 4):V-VI, 1994).
Spawning takes place at 15-25°C, mainly at 17-23°C,
at higher temperatures than A. gueldenstaedtii (8-18°C).
Spawning sites are gravel, pebble, clay or shell beds, depths are 2-20
m and current speeds 1.0-1.7 m/sec. Catches of what were probably this
species in the estuary of the Safid River for the period
1928/29-1936/37 showed strong peaks in April and May with a minor peak
in September and October (Vladykov, 1964). The Safid is the main
spawning river in Iran (Aslaanparveez, 1993). Spawning takes place in
southern Caspian rivers from April to June and again in August to
September. There is a 2 month interruption in spawning in the Safid
River during summer when water temperatures are 26-30°C.
There is a period of at least 2-4 years before this species can spawn
again. Incubation takes 3-5 days. Shafizadeh and Parivar (1999) state that most
embryos hatch 82-87 hours after fertilisation, most of the yolk is absorbed 6
days after hatching and swimup fry appear from day 7 to 8 at 19-21°C. The timing
of passage of fingerlings into the sea after a hatchery release into the Tajan
River was found to be 12-72 hours after release with a peak migration at 0-3
a.m. Smaller fingerlings stayed longer in the river before leaving (Ramezani,
2003).
Egg size is positively correlated with larval length and weight, yolk sac
volume, hatching time and duration of hatching time, but there was no
correlation with mortality during yolk sac absorption or with mortality during
the first feeding stage (Nazari et al., 2009). Imanpoor et al.
(2009) found the average hydrated egg diameter was 3.64 mm, yolk diameter was
3.26 mm, surface-to-volume ratio was 1.65 and yolk sphere-to-perivitelline space
ratio was 0.75, the latter two being very high. The metabolic rate was low and
spawning can occur in low-temperature waters.
Asadi et al. (2006) have examined serum biochemical parameters that
can be used assessing maturity and managing endangered species.
Parasites and predators
Mokhayer (1976b) reports gas bubble disease in Iranian sturgeons
without specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Most of the data for parasites and diseases summarised under A.
gueldenstaedtii above for Iran may well refer to this species. Soltani et
al. (2000) examined parasites of this species in three locations in Gilan
and found Cucullanus sphaerocephalus and Skrjabinopsolus semiarmatus
had the highest prevalence and intensity. Eustrongylides excisus, Anisakis
sp. and Amphilina foliacea were recorded for the first time from this
sturgeon and diet was strongly correlated with diversity of parasites. Soltani and
Kolbassi (2001) describe the use of different antigens for fingerlings against Aeromonas
hydrophila septicaemia.
Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in
juveniles at a frequency of 5.83%.
Hajimoradloo and Ghorbani Nasrabadi (2003) found the prevalence of metazoan
parasites in juveniles of this fish in the southeast Caspian Sea to be 10
species with Anisakis larvae the highest at 19.7%.
Pazooki and Masoumian (2004) report on blood parasites form fish caught at
Anzali, recording Cryptobia acipenseris and Haemogregarina acipenseris.
These parasites caused no pathological effects in the wild fish but can lead to
severe infections and cause anaemia on fish farms. Gorogi (2006a) recorded the
nematode Cucullanus sphaerocephalus, the the digenean Skrjabinopsolus
semiarmatus and the acanthocephalan Leptorhynchoides plagicephalus
from Iranian waters. Sattari and Mokhayer (2005a; 2005b) recorded the occurrence
of parasites in this species from the Iranian southwestern and central coast of
the Caspian Sea. The species found were the nematodes Cucullanus
sphaerocephalus, Eustrongyloides excisus and Anisakis sp., the
cestode Amphilina foliacea, the acanthocephalan Leptorhynchoides
plagicephalus, the digenean trematode Skrjabinopsolus semiarmatus,
the monogenean trematodes Diclybothrium armatum and Nitzschia
storionis and the crustacean Pseudotracheliastes stellatus. General
conclusions were that the diversity of parasites was less in Iranian waters than
in the northern Caspian Sea, perhaps a reflection of the more varied habitat,
its productivity and the carbonate ions differing between the two regions. The
diversity of parasite seems to have declined over time also, perhaps as a result
of unfavourable environmental conditions, particularly in the freshwater
ecosystem which limits the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Diplostomum
spathaceum, Trichodina sp. and Gyrodactylus sp.
Ebrahimi and Malek (2007) found the helminths Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus and
Eustrongylides excisus.
Haghparast et al. (2007) found Cucullanus sphaerocephalus and
Skrjabinopsolus semiarmatus to have the highest incidence (80 and 55%) in
digestive tracts of broodstocks. Masoumzadeh et al. (2007) examined
broodstocks and found Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Eubothrium acipenserinum, Corynosoma
strumosum, Leptorhynchoides plagicephalus and Amphilina foliacea.
Rajabpour et al. (2008) recorded helminth parasites from fish at three
coastal stations in the southeast Caspian Sea, namely the nematode Cucullanus
sphaerocephalus and the digenean Skrjabinopsolus semiarmatus.
Jalilpour et al. (2009) identified a wide range of fungi on eggs and
larvae of fish from the Shahid Beheshti Sturgeon Rearing Centre.
Bazari Moghaddam et al. (2010) examined larvae and fingerlings in the
Shahid Beheshti Hatchery and observed development of parasitism from the ciliate
Trichodina reticulata and the digenean trematode Diplostomum
spathaceum after release into earthen ponds and the river respectively.
Economic importance
See also under A. gueldenstaedtii where much of the data on
this species is subsumed. The average weight of eggs in this species
in Iran is 4-6 kg per fish and these eggs are ideal for first grade
caviar (Vladykov, 1964). This species has the largest abundance (61.9%), biomass
(50%) and catch-per-unit-effort among all Acipenseridae in Iran in both
2003 and 2004 from sampling 85 stations at 2-100 m depths (followed by A.
stellatus (Iranian Fisheries Research Organization Newsletter, 38:1, 2004)).
Catches of A. persicus declined in the Safid River after
construction of a dam at Manjil which released water for rice farming
and held back sediment, both important triggers for attracting
spawning sturgeon. In 1962, flow was reduced to 7-10 cu m/sec
resulting in water temperatures up to 29°C,
destroying insects and crustaceans on which young sturgeon fed and
making the river narrow and shallow (Vladykov, 1964). Many fish were
attracted into the stronger flow of irrigation canals where they
eventually died. Catches of this and other species also declined
because of the introduction of the more efficient synthetic fibre gill
nets in 1957 (Vladykov, 1964). In Iran this sturgeon is caught both in
the sea and in rivers.
Catches in the Safid River in 1930/31-1934/35 peaked at 13,867 fish
in April with 10,693 fish in May and 3433 fish in March and an annual
total of 32,700 fish (Berg, 1948-1949). Holmes (1845) and Eastwick
(1864) reported on fishing for sturgeon in the Safid River. The
principal method in the first half of the nineteenth century was to
stretch 100 foot (30.5 m) lines across the very shallow, rapid and
murky river with 1 yard (0.9 m) lengths of line attached at intervals
of about 2 feet (0.6 m). These lengths of line were armed with large
hooks which snagged the migrating sturgeon. Sturgeon up to 5 feet (1.5
m) were caught from February to April. At the beginning of February
about 100 fish were taken each day, rising to 600-800 at the end of
the month, 800-2000 in March and to 3500-3800 per day in April. After
May sturgeons had little or no roe. About 125,000 fish were taken
annually and sold for their flesh, caviar and isinglass.
Keyvanfar and Nasrichari (1999) state that from an average 2000 t annual
catch over 10 years (1980-1990) 25% of meat and 24% of caviar were from this
species while 17% of meat and 14% of caviar were from A. gueldensatedtii.
This species produces 51% of Iran's caviar production (I.F.R.O. Newsletter,
30-31:5, 2002). Catches in the Kura River from 1974-1978 varied from 90 to 220 tonnes.
Salehi (2011a) summarises the stock enhancement through fingerling release over
the previous two decades in Iran. For the period 2000-2004, for example, 78.7%
of fingerling production were this species.
Extensive studies have been carried out on this species, either on hatchery specimens
to improve their survival or using hatchery specimens as experimental organisms. These
studies include rearing using earthworms (Kazerooni Monfared, 1995); ideal stocking densities in tanks (Derakhshandeh Ghazi
Mahale, 1997); stress during transport and confinement of brood stock as
evaluated using blood samples (Bahmani et
al., 2000; Bahmani and Oryan, 2004); on growth performance
using Daphnia magna and Artemia nauplii as food for fry (50% Artemia and 50% Daphnia given at 70% larval body weight
was the best), and on osmoregulation during restocking (Jabbarzadeh Shiadeh et al.,
2000); procedures against infectious diseases using antigens
from Aeromonas hydrophila which causes septicaemia (Kalbassi et al.,
2000); on effective stocking density of eggs and larvae in incubators and
rearing tanks (Mohseni et al., 2000); haematological variables in juveniles and adults at different water
temperatures (Pourgholam and Saeidi, 2000); on optimum feeding rate for
fingerlings (Yousefpour Pirbazari et al., 2000); on blood parameters for
fingerlings in a Gilan fish farm (Shahsavani et al., 2001); a
histological study of the intestines (Sheibani and Pousti, 2001); sperm has been cryo-preserved to conserve the gene pool (Vecsei
and Artyukhin, 2001); on clove oil having no significant difference with MS222,
an anaesthetic used in fish farms (Abtahi
et al., 2002; 2003); food and feeding of fingerlings after release and
their travel time to the estuary (Kamali and Imanpoor, 2002); the relation
between biochemical composition of eggs and their fertilisation rate (Mohammad
Nazari et al., 2002); changes in the levels of sex steroids as oocytes
developed (Nazari et al., 2002); nutrition in fish ponds where cladocerans and chironomids were staples and copepods and their nauplii were
secondary items (Aslan Parviz and Aghaei Moghadam, 2003; Aghaei Moghadam and Aslan Parviz,
2006); the enhancement effect of ozone and physical treatment on the hatching
rate of eggs (Ghomi et al., 2003); purification and partial
characterisation of serum immunoglobulins (Kalbassi et al., 2003);
physiological studies on the liver oxidase system (Karimzadeh et al.,
2003); toxicity of the insecticide diazinon to fingerlings (Pazhand et al.,
2003); dietary levels of fat and protein effecting growth and chemical
composition of fingerlings (Ebrahimi et al., 2004; Mohseni et al.,
2007); on sperm motility (Hadi
Alavi et al., 2004); the identification of fatty acids in
the flesh and the effects of long-term freezing on them (Hedayatifard and Moini,
2004); effect of temperature on fertilisation percentage achieved by broodstock (16.1-18.0ºC
was optimal)(Hosseini Najd Gerami and Hajimoradlu, 2004); the effect of the timing of first feeding with live food on growth and
survival of larvae (Kordjazi et al., 2004); determination
of the 96h LC50 of Saturn, a herbicide, and Malathion, an
insecticide, at 0.007 and 10 mg/l respectively (Nezami et al., 2004);
histology of the gut from hatching to 56 days (Pahlavan Yali et al.,
2004); levels of zinc and copper in muscle tissue and caviar (Sadeghird et al.,
2004); reproductive conditions of broodstock and when they should no longer be
used (Hosseini Najdegrami et al., 2005); the optimal weight and length
for release of fingerlings into rivers and estuaries (1.8-2.4 g, 6.2-7.5 cm,
33-35 days after yolk sac absorption)(Kazemi et al., 2005); the timing of initial feeding
in relation to behaviour (negative phototaxis and assumption of a benthic life
at 5-6 days post-hatching) and expulsion of the melanin plug (larvae can feed
with it present so expulsion cannot be used to determine active
feeding)(Kordjazi et al., 2005); sperm density and fertilisation
rate (Nazari et al., 2005); the toxic effects
on fingerlings of various pollutants such as the oil products phenol and
1-naftol, the herbicide butachlor, and polyaromatic hydrocarbons from oil wells
in the Caspian Sea (Nezami et al., 2005; Padjand et al., 2005;
Soltani et al., 2006); a macroscopic and microscopic study of the spleen
and and associated lymphatic tissue (Sheibani, 2005); evaluation
of hydrogen peroxide against malachite green (possibly toxic and teratogenic)
for fungal disinfection of eggs showed the former to be superior (Vahabzadeh
et al., 2005); antifungal studies on eggs comparing the
utility of formalin, malachite green and potassium permanganate in fish farms,
the latter being safest for controlling Saprolegnia (Abtahi et al., 2005); sperm studies evaluating ionic composition and osmolality
of seminal plasma, sperm density and motility in regard to sperm
cryopreservation (Alavi et al., 2006); inulin-like growth factor-I
inducing oocyte maturation (Bahrami Kanagar et al., 2006); the micro-cesarean method of extracting
eggs from brood stock was better than conventional methods (Feyzbakhsh et al.,
2006);
the use of rotifers (Brachionus plicatilis) in conjunction with
Artemia nauplii as food for larvae (Haddai Moghadam, 2006); use of oxolinic
acid bioencapsulated in Artemia urmiana as a means to increase resistance
to Aeromonas hydrophila infection in larvae (Hajimoradlou and Agh, 2006); studies on blood serum osmotic and ionic regulation in wild adults and
reared juveniles, important in understanding the best use of water with
different salinities in commercial rearing of this species (Kazemi et al.,
2006); induction of ovulation using glycerin as a solvent
for hypophysis powder proved better than physiologic serum (Noroozi et al.,
2006); feeding
formulated diets to larvae and juveniles in hatchery rearing (Pourali Fashtomi
and Mohseni, 2006); establishing blood serum parameters as tools in disease prognosis
and control (Shahsavani et al., 2006a, 2006b); the maximum allowable concentration of Safid
River sediments as determined in aquaria was 1536.74 mg/l (Yosefi Garakoei et al., 2006);
Abedian Kennari
et al. (2007) on use of Daphnia magna enriched with cod liver oil as
a source of highly unsaturated fatty acid on growth, survival, stress resistance
and fatty acid composition of larvae; the effect of stripping frequency on ionic
content and osmolality in seminal plasma composition (Alavi et al.,
2007); details of sperm morphology in comparison to that of fil mahi (Baradaran
Noveyri et al., 2007); comparison of the efficiency of the Yushchenko and
Azarakhash incubators, the latter being better in terms of fertilisation
percentage, mortality rate, active feeding and survival (Farabi et al.,
2007); ability of Artemia urmiana to act as a carrier of oxolinic acid, a
drug used to combat infection in fish larvae (Ghorbani et al., 2007); use
of probiotic bacillus bioencapsulated with Artemia urmiana nauplii to
increase growth of larvae (Jafarian et al., 2007); fatty
acid composition in fresh and frozen tissues, concluding cold storage should not
exceed 12 months (Moeini and Hedayatifard, 2007);
variations in meat quality using dry and mix salting (salt and 1% madder)
(Seyfzadeh et al., 2007); propagation efficiency of broodstock from two
farms in Mazandaran and Golestan were shown to be different (Yousefian and
Farabi, 2007);
Askarian et al. (2008) examined the gastrointestinal tract for lactic
acid bacteria and found the population levels to be significantly lower than in
Huso huso;on amino acids in food pellets increasing consumption (Jafari Shamushaki et al.,
2008); fertilising ability of cryopreserved spermatozoa (Alipour et al.,
2009); on serum biochemical parameters (Asadi et al., 2009); on the median lethal concentration of suspended sediment from the Safid
River, this pecies showing higher tolerance than A. stellatus (Garakouei
et al., 2009);
isolation of Lactobacillus species, which ferment carbohydrates, from the
intestine (Ghanbari et al., 2009); changes in fatty acid composition after freezing and long-term cold
storage (Hedayatifard and Keyvan, 2009); use of Artemia urmiana enriched
with the essential fatty acid docosahexaenoic acid and its effects on growth,
survival and composition of larvae (Hafezieh et al., 2009); the important
influence of temperature on hatching time, start of exogenous feeding, growth
performance and survival of larvae (Jalali et al., 2009);
immunolocalisation of gill chloride cells used in ionic and osmotic regulation
(Khoushnoud et al., 2009); varied effects of egg size on length, weight
growth and survival of prelarval and early feeding stage (Nazari et al.,
2009); recommended use of methyl paraben as a safe preservative in caviar
infected with the bacterium Clostridium botulinum (Salmani et al.,
2009); regulation of water temperature during the embryonic period, temperatures
of 15-18ºC being the upper limit of thermal
optima (Soleymani and Karimabadi 2009); fish effects of
cooking methods on the physico-chemical and nutritional and digestibility
properties of fillets (Alipour et al., 2010); identification of 13 fungal
species in cultivated and natural populations (Firouzbakhsh et al., 2010); positive
effects of Artemia urmiana enriched with highly unsaturated fatty acids
on growth, survival and fatty acids composition of larvae (Hafezieh et al.,
2010); the effects of sex steroids on hormonal control of reproduction (Hajirezaee
et al., 2010); anaesthetic effects of clove essence (400 p.p.m. and 24ºC
was best treatment and for recovery) (Imanpoor et al., 2010); the impact of plasma sex steroids on gonad development
(Nazari, 2010); the successful use of the synthetic hormone LHRH-A2
on artificial propagation (Nazari et al., 2010): the relationship between steroid hormones and maternal
characteristics and larvae (Nazari and Ghomi (2010); interrelationships among
egg, larvae and maternal characteristics (Nazari et al., 2010); live feed
effects on growth rate of fingerlings (Yousefian et al., 2010); serum
biochemical parameters for disease monitoring and sublethal hatchery conditions
(Yousefian et al., 2010); the growth performance, survival rate and
resistance to thermal stress of Artemia nauplii bioencapsulated with
ergosan (Ahmadifar et al., 2011); the increase in growth efficiency and survival
rate of larvae fed Daphnia magna bioencapsulated with probiotic bacilli (Faramarzi
et al., 2011); growth and changes in fatty acid levels during early
larval development (Babaei et al., 2011); inhibitory effects on lipid oxidation (or rancidity) of ascorbic
and citric acids compared with vacuum packaging in frozen fillets; toxicity of
the herbicide glyphosate (Filizadeh and Rajabi Islami, 2011); bioencapsulated Daphnia magna with yeast product enhancing resistance
against stress and disease of larvae (Lashkarbolouki et al., 2011); the
effect of various feeds on growth and survival of larvae (Lashkarbolooki et
al., 2011); ultrastructure and
osmoregulatory function of larval kidneys (Taghizadeh Rahmat Abad et al.,
2011); on Lactobacillus species from the guts
(Ghanbari and Jami, 2011b); bioencapsulated Daphnia with yeast promoted
feeding parameters and growth in larvae (Jafaryan and Soltani, 2012); the effect of photoperiod on egg hatching rate with soem dark conditions being necessary (Kazemi et al., 2011); sex chromosomes were not differentiated sufficiently
using AFLP to identify males and females at an early life stage (Yarmohammadi
et al., 2011); use of LHRH-a to stimulate immature broodstock to mature
(Amini et al., 2012); a yeast-enriched Daphnia diet increases growth
and feed efficiency, and stress tolerance, in larvae under unfavourable
environmental conditions (Lashkarbolouki et al., 2012);
use of cytochrome
P450 1A in this sturgeon as a biomarker for impact assessment of polycyclic
hydrocarbons pollutants (Karimzadeh et al., 2012); the effect of starvation
and re-feeding on compensatory growth performance and on insulin and blood serum
values in juveniles (Yarmohammadi et al., 2012); etc.
Conservation
See also under A. gueldenstaedtii. Catches in the sea off
Iran are made with large seines and gill nets and many juveniles and
fish below legal size are taken. Netting of sturgeon along the coast of Iran has
been banned and hatchery production in Iran is directed to this species to
maintain stocks. Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 54% for this species among sturgeons captured. Moghiem
(2003) found that catch-per-unit-effort fluctuated from 2.249 to 2.971 kg over
the previous decade, mean length, weight and age declined, the age structure
changed with younger fish increasing in numbers, and catches showed an increase.
Alavi et al. (2005) found overfishing of females in their sample from the
Turkman Sturgeon Fishery Station.
Abdolhay et al. (2006) report on 1062 adults caught in 1998 of which 581
fish were injected with hypophysis extract and produced 22.5 million fingerlings
while in 2002, 802 were caught and 538 produced 12.3 million fingerlings.
Yousefian et al. (2010)found that river water mixed with well water,
saturated with oxygen and maintained at 18±0.2°C
were the best conditions for artificial propagation. The samples used weighed
14-52 kg and an average of 52,000 eggs per female were produced. Fertilisation
was 65 and 75% in two farms, significantly different, and hatching rate was 61%
in both farms. Fungal infections were a significant problem
Hormonal studies are used to select fertile broodstock to
ensure effective aquaculture (Mojabi et al., 1999; Safi et al.,
1999) and other studies relevant to hatchery success, and thus conservation, are
listed above. Nezami
et al. (2000) maintain that sea-ranching has restored this species in Iran.
Moghim et al. (2001) have used ultrasonography to determine sex and
maturity of this species as there are no obvious external sex characteristics.
Sex and maturity determination were accurate at 100% and 98.6% respectively,
confirmed by necropsy, and thus would prevent the loss of male and immature
female fish if the technique were used in the caviar fisheries.
This species is now found in the northern Caspian Sea, the fish being from
Iranian stocking programmes (Kottelat and Freyhof, 2007).
Amini (2005) and Abdolhay and Tahori (2006) summarise hatchery production for this species:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured
|
661 |
591 |
620 |
2056 |
742 |
| Injected broodstock |
437 |
492 |
528 |
1288 |
436 |
| Spawning rate* (%) |
81 |
86.5 |
410 (sic) |
80 |
85 |
| Fertilisation rate (%) |
72 |
76.1 |
83 |
71 |
75 |
| Survival rate in incubators
(%) |
56 |
52.6 |
75 |
50 |
64 |
| Survival rate in tanks (%) |
76 |
76.4 |
53 |
67 |
74 |
| Stocking density in ponds
(fish/ha) |
84,076 |
89,131 |
76,000 |
97,941 |
95,661 |
| Survival rate in ponds (%) |
56 |
47.4 |
56 |
52 |
56 |
| Fingerling production |
13,711,199 |
16,278,595 |
12,301,214 |
18,388,962^ |
17,412,529 |
* Rate of response to hormone injection; ^ 18,288 in Abdolhay and Tahori
(2006)
Studies on heavy metal contamination (Zn, Cu, Cd,, Pb and Hg) of both flesh
and caviar showed levels were below the maxima allowed for consumption, based on
international standards (Sadeghi Rad et al., 2005; Amini Ranjbar et al.
(2003), Amini Ranjbar and Shariat, 2006; Sadeghi Rad et al., 2009).
Lelek (1987) lists this species as endangered.
Extinct in Turkey (Fricke et al., 2007). Kiabi et al.
(1999) consider this species to be vulnerable in the south Caspian Sea
basin according to IUCN criteria. Vecsei and Artyukhin (2001) list it as
endangered with the IUCN. Criteria include commercial fishing,
abundant in numbers, habitat destruction, widespread range (75% of
water bodies), absent in other water bodies in Iran, and present
outside the Caspian Sea basin. Mostafavi (2007) lists it as vulnerable in the
Talar River, Mazandaran. Kottelat and Freyhof (2007) state that there is
likely no natural reproduction in Iranian waters, fish being from artificial
stocking programmes.
Further work
Fresh samples of sturgeon from Iranian rivers should be examined
systematically and with care to determine if they are indeed this
species and not A. gueldenstaedtii. A detailed comparative
study of the morphology of this species and Acipenser
gueldenstaedtii in Iran would enable the young and adults to be
clearly distinguished as well as stocks within each species as a management tool.
Sources
Holcik (1993) and Shariati (1994) give accounts of this species in Farsi. See also under family above.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Acipenser ruthenus
Linnaeus, 1758
Found in the Caspian Sea basin but no records from Iran proper.
Single specimens have been recorded as entering the Kura River of
Azerbaijan and fishermen reported one fish from off Soviet Astara in
1929 (Berg, 1948-1949) on the border with Iran. The import of 30,000
fingerlings and 20 male parent stock of this species to Iran for
artificial reproduction was envisaged in an agreement with the Russian
Research Centre of Commercial Sturgeon Reproduction in 1995 (Iranian
Fisheries Research and Training Organization Newsletter, 9:3,
1995). Tatina et al. (2010) studied effects of dietary vitamins C and E
on haematological and biochemical parameters in this fish in the breeding centre
in Rasht. Acipenser primigenius Chalikov, 1944 is a hybrid of this
species and Acipenser gueldenstaedtii (Eschmeyer et al., 1996).
The Farsi name is استرلياد (esterliad).
Listed as Endangered in the Volga River (Peterson et al., 2009).
Acipenser stellatus
Pallas, 1771

Common names
ازون برون or اوزون
بورون (uzun burun or ozoonboroon = long nose),
دراكول (= derakul or darakul); tirij
(after Wossugh-Zamani (1991a), meaning shaped like an arrow; see also A. persicus);
سوروگا (= sevruga or sevroga), سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), puze draz.
[uzunburun, Kur uzunburun for natio cyrensis, ag-balyk, all in
Azerbaijanian; tirana in Turkmenian; sevryuga, sevruga or stellate sturgeon (this term also
includes A. nudiventris with small eggs for fisheries
statistics), yuzhnokaspiiskaya sevryuga or South Caspian stellate
sturgeon, both in Russian; star or starred sturgeon].
Systematics
Originally described from the Volga River near Simbirsk.
Acipenser seuruga Güldenstädt, 1772 from the Caspian Sea, Acipenser
hellops Pallas, 1814 from the Black and Caspian seas, Acipenser
Helops Pallas, 1814 from the Araks River, and Acipenser
Ratzeburgii Brandt in Brandt and Ratzeburg, 1833 from the Caspian Sea at the
mouth of the Emba River, are synonyms.
Acipenser stellatus stellatus natio cyrensis Berg, 1932
is described from the southern Caspian Sea and tributary rivers but
has no taxonomic status as an infrasubspecific rank. Morphologically,
this Kura River form is similar to north Caspian members of the
species, differing principally in postorbital distance. Growth and
fecundity are lower in the Kura form and spawning time is different.
M. Poorhazemi (Pourkazemi) finds that A. stellatus is highly polymorphic
with more than one population using molecular techniques (Iranian
Fisheries Research and Training Organization Newsletter, 14:4-5, 1996).
Norouzi et al. (2009) used microsatellite markers to determine that there
is more than one population in the south Caspian Sea which has importance in
terms of stock management, restocking and conservation. Shabani et al. (2003; 2006) found no significant differences between
Volga River and Gorgan, Tajan and Safid River fish of Iran when examining mtDNA.
Norouzi et al. (2008) and Norouzi and Pourkazemi (2009) examined the
population and genetic structure of this species in Iranian waters using
microsatellite markers and found evidence for at least three populations,
particularly a separate one in the Safid River, and probably more than one in
each river such as the Safid and Gorgan rivers.
A hybrid with Acipenser nudiventris is reported from the
Safid River (Nedoshivin and Iljin, 1927). Artificial hybrids with Huso
huso have been produced in Mazandaran for aquaculture projects (Annual
Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran, p. 6, 1996).
Key characters
This sturgeon has a long snout (59-65% of head length) with a
pointed tip in contrast to the short snout and rounded tip in A.
gueldenstaedtii and A. persicus. The continuous lower lip
in A. nudiventris and the large crescentic mouth in Huso
huso distinguish these species.
Morphology
The lower lip is interrupted at its centre, barbels are not
fringed, are short, and do not reach the mouth but are closer to the
mouth than the snout tip.
Dorsal fin rays 38-54 and anal fin rays 20-40; or 40-54 and 22-35
respectively in the Kura for natio cyrensis (Berg, 1948-1949).
Dorsal scutes 9-16, lateral scutes 26-43 and ventral scutes 9-14.
There are smaller scutes between the main rows. Gill rakers 24-29,
usually 25-26 in natio cyrensis. Chromosome number 2n=115 ± 1
(Annual Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 43, 1996), 2n=118 ± 2 or 113 ± 1 (Iranian
Fisheries Research and Training Organization Newsletter, 8:5,
1995) or 2n=118 ± 1 (Nowruz Fashkhami, 1996), 2n=114 (Nowruz
Fashkhami and Khosroshahi, 1999); 2n=146±6 (Chicca et al., 2002).
Sheibani (2003a) described the anterior digestive canal of this species.
Keyvanfar (1986) found a transferrin polymorphism in the serum
proteins of this species but not the other Iranian species of sturgeon and
Keyvanfar (1988) found several variants corresponding to
transferrin in the other species.
Sexual dimorphism
Females are larger than males of the same age; in the Ural River
1.3-1.6 times larger. Head depth and preanal distance differ between
sexes in Kura River fish but only when gonads are ripening.
Abdurakhmanov (1962) reports a longer anal fin, snout, and snout tip
to barbel distance in males, and a longer predorsal length, preanal
length, postorbital length and a greater caudal peduncle depth in females.
Colour
The back is dark grey, ash grey or cinnamon brown, almost black in
some fish, and fades to a white belly. Flanks are yellowish-white. In
small fish, the scutes are lighter than the adjacent body and so are
distinctive. Sea fish are darker than river fish. An eyeless specimen,
1.11 m long, caught in Mazandaran was dark black (Abzeeyan,
Tehran 4(7):V). The eyes were completely absent and their position on
the head was covered with smooth bone.
Size
Attains about 2.21 m and possibly more than 80 kg. Sternin and Doré (1993)
cite a specimen of 2.9 m. Iranian captures averaged 1.3-1.4 m and 9-10
kg in the 1950s (Farid-Pak, no date). One of the largest specimens
ever caught was 2.18 m long and was taken off the Astara River on the
border of Azerbaijan and Iran in 1932. Much larger fish are known from
archaeological sites of the 10th-13th centuries on the Terek River, up
to 2.7 m (Tsepkin and Sokolov, 1971).
Distribution
Found in the Adriatic, Aegean, Black and Caspian seas and their
drainages but the largest populations are in the Caspian. Generally
found from the Astara River in the west to the Gorgan River in the
east in Iran (Berg, 1948-1949; Kozhin, 1957; Armantrout, 1980) but not the Atrak River on the eastern
Caspian border of Iran with Turkmenistan (Berg, 1936). Found in the
Safid River at Kisom and the Mirerud (Derzhavin, 1934; Kozhin, 1957). It used
to ascend the Aras River but numbers in Iranian reaches were always small (Berg,
1948-1949). The Kura River catch was up to 90% of the sturgeons taken. Rostami
(1961) records this species from several localities on the Safid River and from
the Golchan, "Djef", Youssefabad, Tchontchenan, Dehkah, Sorkh, Talar,
Tajan, and Neka rivers. Also
reported from Kargan and Hasan Kiadeh by V. D. Vladykov based on field
work notes made in 1962. Reported more recently from the Gorgan,
Gharasu, Tajan, Babol, Haraz, and Safid rivers, Gorgan Bay, the
southeast Caspian Sea, southwest Caspian Sea and south-central Caspian
Sea by Kiabi et al. (1999) and Abdoli and Naderi (2009) and from the Safid River and Anzali Talab by Abbasi et al. (1999).
Zoogeography
Presumably a relict of the past isolation of waters now
encompassing the Black-Caspian seas.
Habitat
This sturgeon is found in large concentrations in the eastern
coastal region of the south Caspian Sea in August-September with up to
25-30 fish taken in a single trawl, having moved south from northern
waters. Ivanov and Katunin (2001) note the densest concentration in the
per-estuary zone of the Gorgan River, with catches reaching 26 fish/trawl
while along the central part of the Iranian coast catches did not exceed 4
fish/trawl. At the end of winter and particularly in early spring, uzun
burun move onto the Iranian shore. Migrations between the Kura River
lower reaches, the Safid River and elsewhere are reported. They
usually does not descend below 100-130 m except along the southern
shore of the Caspian Sea (Legeza, 1973) where they may descend to 300
m. Uzun burun are common only down to 50 m. There is no seasonal
variation in depth distribution in the south Caspian Sea in contrast
to the middle Caspian. They are often found in surface waters during
the day, and retire to the bottom during the night. Uzun burun are
found on silt and sand-silt bottoms but will also feed on sand and
shell grounds. Temperature range is 4-24°C, in winter 7.5-10.5°C
and 11.0-24.0°C in summer and fall, with an absolute range of 2.4-29.5°C.
Water temperatures below 6°C are unsuitable for feeding however. Salinity range in the sea is
0.1-14.6‰ and this is the most euryhaline sturgeon in the Caspian Sea. This species is the best swimmer among sturgeons in the
Caspian Sea in terms of power to body weight and in the Volga River
migration speed averages 110 km/day (although progress is only 17.6
km/day because of the current).
The effects of diazinon on haematological parameters was examined by
Khoshbavar Rostami et al. (2005) who also found the LC50 was 4.98 mg/l over 96 hours.
Hiedary et al. (2012) found bioaccumulation of copper, zinc and mercury
in muscles and liver, influenced by their concentration in sediments and the
physiological state of the fish. The levels did not constitute a human health
threat.
Age and growth
Maximum age for accidental catches in the Caspian Sea off
Azerbaijan is 21 years but most are 8-13 years old. Males mature at
11-13 years, the youngest at 7 years, and females at 14-17 years, the
youngest at 8 years in the Kura River. Populations in the Kura River
and Iranian rivers take the longest time to mature, have a slower
growth rate and lower fecundity. Vecsei et al. (2007) give a maturity
range of 5-17 years. Like other sturgeons, this species
does not reproduce every year and in the Caspian and there is a 3-4
year gap between reproductive periods in any individual. Females live
longer than males. Maximum life span is about 41 years.
Levin (1997)
summarises the Volga spawning population as being age 6-28 years
(11-16 years on average) with females 150-152 cm and 11-12 kg and
males 128-130 cm and 6-7 kg. Spawning temperature is 16-22°C.
The stock on the
Iranian coast was estimated at 3.2 million fish weighing 18,500 tonnes with 6.7%
of fish mature (Ivanov and Katunin, 2001).
Studies in 2007 along the whole Iranian coast when 50 stations were sampled
in waters less than 10 m deep, found this species to comprise 11.8% of the
absolute frequency and 38.7% of the biomass of the total sturgeon catch, second
after A. persicus (Iranian
Fisheries Research Organization Newsletter, 51:2, 2007).
Von Bertalanffy growth parameters in Iranian females are L
∞ = 213 cm and K = 0.062 or 188 cm and 0.104 and for males 190 cm and
0.083 or 171 cm and 0.113 depending on the methodology used. Total
mortality (Z) was 0.52-1.1 for females and 0.62-1.1 for males, natural
mortality (M) was 0.07 for females and 0.08 for males, fishing
mortality (F) was 1.03 for females and 0.54 for males, and optimum
fishing mortality was (F) 0.42 for females and 0.30 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
Samples taken from the whole Caspian shore of Iran from 2002 to 2004 had growth
parameters ∞ = 219 cm and K = 0.06 year-1
(www.shilat.com, downloaded 28 February 2007).
Yelghi et al. (2007) found maximum age frequencies for fish from the
southeastern Caspian Sea were were 9-13 years for male and 12-13 years for
females. Brood fishes more than 15 years old formed little of the total catch.
The oldest and largest individuals were 17 years and 156 cm for males and 27 years
and 178 cm for females.
Growth was negative allometric. Bakhshalizadeh et al. (2012) found
a maximum age of 29 years for samples from the commercial Iranian fishery from
2008 to 2010. This study also gave the asymptotic length (L∞)
as 153.69 cm and 131.02 for females and males respectively, a growth
coefficient (K) of 0.08 and 0.15 per year, and a total mortality coefficient (Z)
of 0.79 and 1.08 per year. Annual mortality rates were 55% for females and 66%
for males.
Food
Young specimens feed on crustaceans, older fish on chironomid
larvae and the oldest specimens on fish (Rostami, 1961b). Azari Takami
et al. (1980) found adults to consume gobies (Gobiidae) and
kilka (Clupeonella) with the clams Abra ovata and Cerastoderma
umbonatum as secondary items in Iran. In the Caspian Sea off
Azerbaijan, Zarbalieva (1987) found that the polychaete worm Nereis
diversicolor (82.7% by weight) dominated in the diet of sturgeons
20-80 cm long, being replaced by the mollusc Abra ovata (88.6%)
at 90-120 cm and by Clupeonella spp. (65.1%) and Abra ovata
(31.5%) at 125-140. Sturgeons 50-80 cm long also took the crab Rhithropanopeus
harrisii (21.2%). Other foods include Rutilus rutilus (and presumably
R. caspicus) Cobitis
taenia (presumably C. keyvani), mysids, cumaceans, and amphipods. Gobies are generally of
lesser importance than clupeids. Hashemyan et al. (2005) found diet in
A. persicus, A. stellatus and A. nudiventris in coastal waters
of Mazandaran and Golestan at depths less than 20 m to consist of annelids
(50.8%), amphipods (41.5%), small fish 4.8%), decapods (2%) and bivalves (0.9%).
Fish shorter than 40 cm fed mostly on shrimps, polychaetes and gammarids, 41-80
cm fish fed on shrimps, gammarids, polychaetes, bivalves and smaller fish, while
fish greater than 80 cm fed mostly on shrimps and smaller fish. Haddadi Moghadam
et al. (2009) studied diet in fish collected in summer and winter in the
south Caspian Sea from 2004 to 2006. Food items were fishes (Neogobius
sp., Atherina caspia, Clupeonella cultriventris (= caspia) and
invertebrates (polychaete worms such as Ampharetidae and Nereis diversicolor;
crustaceans such as Gammarus and Paramysis; and the bivalve
mollusc Abra ovata). The diet varied with season and size group and was
similar to A. persicus.
In rivers, juveniles feed on gammarids, chironomid larvae, mysids
and worms. Spawning fish eat little or no food and, having used up
much of their fat reserves, return to their feeding grounds in the sea
immediately after spawning. This downstream migration varies from 70 to 80 km/day.
Reproduction
The peak migration in Iran is in April. There is also a peak run in
fall (September-October) in the Kura River, and probably in Iran too
(see below), but it is much less important than the spring run (Berg,
1959). Migrations in the Kura and Safid rivers can be found year round
outside these peaks. The spring run in the Kura begins at about 10°C
and peaks at 18°C, the runs decline in warmer summer temperatures and the fall run begins
as water cools. Water level is also an important factor influencing
runs and spawning. Water level fluctuations exceeding 0.2-0.5 m causes
spawning to stop as fish migrate to deeper water. Summer and fall run
fish do not spawn until the following year. Males arrive on the
spawning ground before females and stay up to 6 weeks; females stay
only 10-12 days. The Volga run begins in March-April with a peak in
May but continues to October-November (Levin, 1997).
Up to 950,000 adhesive eggs are laid although in rivers of the
southern Caspian absolute fecundity is lower, 35,400-362,900 eggs in
the Kura River for example. Fertility is higher in the Volga compared
to the Safid River (Iranian Fisheries Research and Training
Organization Newsletter, 17:6, 1997). The spawning period in the
Kura River is April-September at 15-29°C.
Fish may leap out of the water during spawning and scrape their bodies
on the bottom, leaving scratches and bruises. Eggs are deposited over
gravel, pebbles, or stones mixed with shell fragments and coarse sand
in the river bed or on flooded banks at a current velocity of 0.7-1.8
m/sec. A gravel bottom and a current speed of 1.2-1.5 m/sec are ideal.
Eggs are round to ovate, brownish-grey and up to 3.2 mm in diameter.
The adult loses 25-30% of its weight after spawning and females are
only ready to spawn again after 5-6 years and males after 3-4 years. Spawning
occurs at 15-26°C. Incubation takes 44-80 hours at 20-28°C.
Young fish descend to the sea at 3-4 months of age but in some
populations this occurs immediately after hatching, taking only 12-15 days.
Moghim et al. (2000) have used ultrasonography to determine sex and
maturity stage of this sturgeon. Sex determination had a 97.2% accuracy and took
30 seconds or less per fish. This non-invasive technique reduces stress and
enables immature females caught at sea to be released.
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) record the acanthocephalan Leptorhynchoides
plagicephalus and describe a new species, Corynosoma caspicum,
from this sturgeon in Iran. The coelenterate Polypodium hydriforme
is recorded from the eggs of this sturgeon in the Safid Rud. Mokhayer
and Anwar (1973) report on sturgeon parasites in general (see under Acipenser
gueldenstaedtii). Mokhayer (1976b) also reports gas bubble disease
in Iranian sturgeons without specifying the species of sturgeon as
well as the monogenetic trematodes Diclobothrium armatum and Nitzschia
sturionis. Larvae of the nematode Anisakis is reported from
this species in Iran (Eslami and Mokhayer, 1977). Mokhayer (1989)
reports metacercariae of the eye fluke, Diplostomum spathaceum
from this species in Iran, which can cause complete blindness and
death in commercially important species.
Sattari et al. (2001) found the following parasites in fish from the
southwest Caspian Sea: Skrjabinopsolus semiarmatus, Leptorhynchoides
plagicephalus, Cucullanus sphaerocephalus, Eubothrium
acipenserinum, Bothriomonus fallax, Eustrongylides excisus,
Aniskais sp., Amphilina foliacea and Corynosoma strumosum. Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in adult fish.
Pazooki and Masoumian (2004) report on blood parasites form fish caught at
Anzali, recording Cryptobia acipenseris and Haemogregarina acipenseris.
These parasites caused no pathological effects in the wild fish but can lead to
severe infections and cause anaemia on fish farms. Sattari and Mokhayer (2005a;
2005b) recorded the occurrence of parasites in this species from the Iranian
southwestern and central coast of the Caspian Sea. The species found were the
nematodes Cucullanus sphaerocephalus, Eustrongyloides excisus and
Anisakis sp., the cestodes Eubothrium acipenserinum, Amphilina
foliacea and Bothrimonus fallax, the acanthocephalans
Leptorhynchoides plagicephalus and Corynosoma strumosum, the digenean
trematode Skrjabinopsolus semiarmatus. General conclusions were that the
diversity of parasites was less in Iranian waters than in the northern Caspian
Sea, perhaps a reflection of the more varied habitat, its productivity and the
carbonate ions differing between the two regions. The diversity of parasite
seems to have declined over time also, perhaps as a result of unfavourable
environmental conditions, particularly in the freshwater ecosystem which limits
the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Diplostomum
spathaceum, Trichodina sp. and Gyrodactylus sp.
Ebrahimi and Malek (2007) found the helminths Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus and
Eustrongylides excisus.
Rajabpour et al. (2008) recorded helminth parasites from fish at three
coastal stations in the southeast Caspian Sea, namely the nematode Cucullanus
sphaerocephalus, the digenean Skrjabinopsolus semiarmatus, the
acanthocephalan Leptorhynchoides plagicephalus and the cestode
Amphilina foliacea.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
Predators are most evident on the young and include Silurus
glanis and various gobies (Gobiidae) while eggs are taken by Blicca
bjoerkna, Pelecus cultratus, Gobio sp., and gobies.
Economic importance
Uzun burun are known from a Neolithic site on the eastern Caspian
shore in the former Soviet Union from about 6000 years ago (Tsepkin, 1986).
This sturgeon provided the majority of the caviar produced in Iran
according to reports from the 1960s and beginning of the 1970s (Vladykov,
1964; RaLonde, 1970b), 70% of the total catch according to commercial
suppliers in 1995. It is reputed to have the tastiest flesh and also
the best caviar (Ricker, 1970) but others maintain beluga caviar is
the best. Farid-Pak (no date) gives an average yield of 1.5-2.0 kg for
each female in the 1950s in Iran. Catch records for the Safid River in
1930-1935 showed that 31.7% of fish were caught in May, 18.1% in April
and 9.6% in June, with a small peak in October of 7.9%. Nevraev (1929)
records catches of this species varying from 22,278 to 43,593
individuals in the Astara region of Iran for the period 1901-1902 to
1913-1914, for the Safid Rud region 5536 to 12,670 individuals for the
period 1899-1900 to 1913-1914, for the Mazandaran region 846 to 1490
individuals for 1906-1907 to 1913-1914, and for the Astrabad (= Gorgan)
region 2613 to 5160 individuals for 1902-1903 to 1913-1914. Vladykov
(1964) records average yearly catches in Iran of this species
(including some A. nudiventris with small eggs) from
1927/28-1931/32 to 1957/58-1961/62 with ranges of 59,291-301,218 kg
body weight (9.7-23.8% of the total sturgeon catch; 33.8% in another
five-year period when weight was lower than the maximum shown here)
and 8246-77,780 kg caviar (10.0-48.2%; total range 9.5-54.5%). RaLonde
and Walczak (1970b) summarise yields for the years 1963 to 1967 in
Iran of meat and caviar as 385.2 tonnes (100.4 tonnes), 450.8 (99.3),
436.6 (98.9), 564.4 (113.0), and 584.7 (106.5) respectively. Hassan
Nia (1995) analysed the stocks of this species for a 61-year period
(1927-1987) and calculated projected yields for the period 1988-1992.
Actual yields proved to be the same as projected yields. The catch in
the northern Caspian Sea reached 13,200 tonnes in the latter half of the 1970s.
This species has not been used as extensively as others for studies on
physiology, biochemistry and aquaculture. Some works include Taleban et al. (1998) who studied consumption of this fatty fish and found
a reduction in mean serum triglycerides and very low lipoprotein cholesterol, and
an increase in high density lipoprotein cholesterol; Pourgholam and Saeidi
(2000) investigated haematological variables in juveniles and adults at
different water temperatures; Hedayatifard et al. (2003) studied
variation in fatty acids composition in cold storage and found the best holding
time was three months; Pazhand et al. (2003) on the toxicity of
the insecticide diazinon to fingerlings; Sadeghird et al. (2004) examined
levels of zinc and copper in muscle tissue and caviar; Padjand et al. (2005) examined the toxic
effects on fingerlings of the herbicide butachlor; Hedayatifard and Moeini (2007)
determined the levels of fatty acids in fresh and
frozen samples and their effects on shelf life; Hedayatifard and Yousefian
(2007) looked at shelf life and changes of lipid and fatty acid composition in
frozen storage; Mokaremi Rostami et al. (2007) on the effects on
juveniles of creosote on mortality rate and blood biochemistry with significant
differences from controls; Alipour et al. (2009) on
fertilising ability of cryopreserved spermatozoa; Bahmani et al. (2009)
on seasonal fluctuations of sex steroids in farmed 7-year-old fish; Asadi et
al. (2009) on serum biochemical parameters; Hedayatifard
and Aroujalian (2010) on packaging and shelf life; Filizadeh and Rajabi Islami
(2011) on toxicity of the herbicide glyphosate; etc.
The use of 2000 p.p.m. potassium sorbate in processing caviar from
this species gives a better quality product than caviar without
preservatives (Salmani, 1995).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria and as food.
Conservation
See also under A. gueldenstaedtii. Lelek (1987) lists this
species as vulnerable and Birstein (1993) as intermediate in status.
It is now rare in the Safid and Gorgan rivers of Iran because of dam
construction, which inhibits the spawning migration, and irrigation
control structures near river mouths. The ban on sea fishing in 1962
by Soviet authorities led to an increased abundance of this species.
Artificial spawning sites with gravel 3-10 cm in diameter have proved
useful in the former U.S.S.R. and stocking is well established with up
to 23 million young being released in the Volga area annually in the
mid-1970s. However Veshchev (1995) reports that the population of this
species in the Volga could be lost, and this doubtless mirrors the
situation in other Caspian Sea states including Iran. About 30% of all
individuals caught in the Caspian in the late 1980s were hatchery
stock (De Meulenaer and Raymakers, 1996).
Abdolhay et al. (2006) report on 193 adults caught in 1998 which produced
623,000 million fingerlings while in 2002, 290 breeders were caught and 67
produced 1.3 million fingerlings.
Mohseni et al., (2000) have studied
effective stocking density of eggs and larvae in incubators and rearing tanks in
order to maximise production and avoid various morphological deformities.
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 37% for this species among sturgeons.
Khodorevskaya et al. (1997) summarises the decline of this
species in the Volga and Ural rivers. The problems are the same for
all sturgeons, namely flow alterations affecting the volume of water
on the spawning grounds, reduction in numbers reaching the these
grounds through poaching, and increased pollution affecting
reproductive efficiency.
Studies on heavy metal contamination (Zn, Cu, Cd,, Pb and Hg) of both flesh and
caviar in Iran, however, showed levels were below the maxima allowed for
consumption, based on international standards (Sadeghi Rad et al., 2005;
Abtahi et al. 2007).
The median lethal concentration of suspended sediment from the Safid River has
been studied by Garakouei et al. (2009); who found this species showed a
lower tolerance than A. persicus.
Kiabi et al. (1999) consider this species to be vulnerable
in the south Caspian Sea basin according to IUCN criteria. Criteria
include commercial fishing, abundant in numbers, habitat destruction,
widespread range (75% of water bodies), absent in other water bodies
in Iran, and present outside the Caspian Sea basin. Nezami et al.
(2000) maintain that despite artificial spawning and fingerling production,
restoration of this species in Iran was not very successful.
Mostafavi (2007) lists it as vulnerable in the Talar River, Mazandaran. Critically endangered in Turkey (Fricke et al., 2007). Under IUCN and Appendix II of CITES, this species is now endangered (Vecsei
et al., 2007).
Artificial breeding has been carried out with this species in Iran using
hormones (I.F.R.O. Newsletter, 30-31:4, 2002). In contrast to other sturgeons,
this species does not respond well to pituitary injections used to stimulate
artificial reproduction. Pourkazemi (2006) examined haematological parameters
and found wide fluctuations, with female spawners in particular differing in
sexual maturity and physiological state. Although fish do respond to pituitary
injections, the oocytes do not follow a normal course to maturity, remaining in
the ovary. Oocytes at stage IV had overripe or degenerated oocytes. When
overdosed with pituitary extract, ovulation occurred but oocytes were not mature
and could not be fertilised. Degeneration of the egg membrane was found in 82%
of spawners caught in the wild, presumably due to pollution. Baradaran Tahouri
(1994) examined the effects of pond fertilisation on growth. Haddadi Moghaddam
et al. (2001) studied the growth rate of this sturgeon in fertilised
earthen ponds with added Daphnia. Shahsavani et al. (2001)
determined blood parameters for fingerlings in a Gilan fish farm. Bahmani et al.
(2006) recommended alleviating stress during capture, handling, transport and
confinement, selecting breeders with suitable morphology and correct stage of
sexual maturity, and using the hormone GnRH with domperidone as a substitute for
pituitary extract. Luteinizing hormone releasing hormone
analogue (LHRHa) was also found to be effective at 20.0-31.2
μg/kg body weight (Behmanesh, 2002).
Kazemi et al. (2003) give a detailed histological study of the oocytes of this species.
Caviar and fingerlings have been produced from farmed breeders (Iranian
Fisheries Research Organization Newsletter, 49:3, 2006).).
Sexual maturity was stimulated by injection of GnRH and anti-dopamine, eggs were
extracted surgically, of which more than 80% hatched successfully using sperm
taken by using tubes, and caviar and flesh harvested from one fish was
comparable to natural samples.Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
101 |
58 |
43 |
70 |
52 |
| Injected broodstock |
43 |
38 |
67 |
42 |
12 |
| Spawning rate * (%) |
60.4 |
50 |
49 |
63 |
50 |
| Fertilisation rate (%) |
55 |
58.6 |
51 |
51 |
83 |
| Survival rate in incubators
(%) |
23 |
49.6 |
46 |
44 |
77 |
| Survival rate in tanks (%) |
54 |
93.5 |
60 |
72 |
77 |
| Stocking density in ponds
(fish/ha) |
92,500 |
44,812 |
68,000 |
90,000 |
92,000 |
| Survival rate in ponds (%) |
12.2 |
67.7 |
38 |
61 |
86 |
| Fingerling production (x
1000) |
226 |
820 |
13,009 |
196 |
314 |
* Rate of response to hormone injection
Moghim et al. (2002) used
ultrasonography to determine sex and maturity. This is important in management
of endangered species when external sexual dimorphism is not apparent. Accuracy
was 97.2% and was around 30 seconds or less per fish.
Further work
See under A. gueldenstaedtii.
Sources
See under the family account. Wossugh-Zamani (1991a) gives an account of
this species in Farsi. Derzhavin (1922) and Borzenko (1942) are older
works giving details of the biology of this species.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Comparative material: BM(NH) 1873.4.21:21-23, 2, 99.6-236.6 mm total length,
Russia, Black Sea (no other locality data); BM(NH) 1929.8.7:4-5 and BM(NH)
1930.3.21:2, 3, 246.4-308.2 mm total length, Ukraine, Sebastopol, Black Sea (no
other locality data).
Genus Huso
Brandt and Ratzeberg, 1833
This genus is characterised by a large and crescentic mouth (small
and transverse in Acipenser) and by the gill membranes being
joined to each other and free of the isthmus (joined to the isthmus in
Acipenser). The snout is short and blunt although Caspian Sea stocks have
a longer snout than Black Sea ones. The barbels are
flattened laterally and gill rakers are rod-like. There are only 2
species in the genus, one in the Caspian, Black and Adriatic seas and
one in the Amur River of eastern Asia.
Birstein and DeSalle (1998) using cytochrome b and 12S and 16S rRNA genes found that Huso may not be
distinct from Acipenser.
Vasil'eva et al. (2009) using cytogenetic and morphological characters
also advocate reverting to the original genus Acipenser for Huso
species.
Huso huso
(Linnaeus, 1758)


Huso huso, Iran, 1959, courtesy of Hamid Niksirat (no other data)Common names
فيل ماهي (= fil mahi, filmahi or
philmahi meaning elephant fish), beluga, beloga, سگ ماهي (sag mahi,
meaning dogfish), ماهي خاويار (= mahi-ye
kaviar, meaning caviar fish), mahi kaviar-e bozorg (= big caviar fish).
[bolka, Kur bolkasi for natio kurensis, ag-kulag-nyarya,
gyuz'gi-burun in Azerbaijanian; doku (akvalyk) in Turkmenian; beluga in Russian; great, giant or European sturgeon].
Systematics
Acipenser huso was originally described from the Danube and rivers of Russia.
Huso huso caspicus Babushkin, 1942 was described as the
subspecies of the Caspian Sea basin (with natio kurensis
Babushkin, 1942 from the Kura River (also spelt incorrectly cyrensis
and curensis)) but Berg (1948-1949) considered Caspian-Volga
populations to be typical and this subspecies description as
unnecessary. No types of Huso huso caspicus are known
(Eschmeyer et al., 1996).
Huso ichthyocolla Bonaparte, 1846 is a synonym (Eschmeyer et
al., 1996) and a nomen nudum (Holčík, 1989).
Acipenser brandtii Günther, 1870 from the "Black
and Caspian Seas, with their rivers" is a hybrid of Huso huso
and Acipenser nudiventris based on Acipenser schypa (in
part) of Brandt and Ratzeberg (Berg, 1948-1949; Eschmeyer et al., 1996).
M. Pourkazemi in PADECO (2002) considers there are two sub-populations in Iran
and Ghadirnejad et al. (2008) using microsatellite loci concluded that
there were possibly two populations in the southern Caspian Sea.
Hybrids of Huso with Acipenser have been bred by the
Aquaculture Department of the Iranian Fisheries Research and Training
Organization (Iranian Fisheries Research and Training Organization
Newsletter, 3:3, 1994; Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 6, 1996; Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization, Tehran,
p. 41, 1997) and natural hybrids with A. gueldenstaedtii, A. nudiventris and A. stellatus are
reported from the Caspian Sea (Berg, 1948-1949).
Yarmohammadi et al. (2012) were able to identify besters (female Huso
and male A. ruthenus hybrids) from the International Sturgeon Research
Institute in Rasht using the AFLP molecular technique.
Key characters
This species is identified by its very large, crescent-shaped mouth
(small and transverse in other sturgeons) and the gill membranes being
joined as a fold across the isthmus.
Morphology
The greatest body depth is slightly anterior to the middle of the body and
large fish appear humpbacked. The lower lip is interrupted at its centre. Barbels are flat
posteriorly, reach almost to the mouth and have foliate appendages.
Experiments on ablading barbels (clipping one, two and four barbels) in 1+ age fish
showed no growth differences with an unclipped control (Abasali Zadeh, 2003).
The dorsal scutes are covered with skin in sexually mature fish,
lateral scutes are smooth and ventro-lateral scutes hidden beneath the skin.
Dorsal fin rays 48-81 and anal fin rays 22-41. Dorsal scutes 9-17,
lateral scutes 28-60 and ventral scutes 7-14. Scutes in adults may be
reabsorbed. The skin is covered in small denticles. Gill rakers 16-36.
The chromosome number is 2n=118 ± 3 or 115 ± 1 (Annual Report,
1994-1995, Iranian Fisheries Research and Training Organization,
Tehran, p. 43, 1996; Iranian Fisheries Research and Training
Organization Newsletter, 8:5, 1995), 2n=116 ± 1 (Nowruz
Fashkhami, 1996), 2n=118 ± 2 or 2n=116 ± 4 (Klinkhardt et al., 1995),
2n=117 (Nowruz Fashkhami and Khosroshahi, 1999). Sex chromosomes are absent or
weakly differentiated in the genome and DNA markers cannot be used to sex
fish; minor surgery has to be used (Keyvan Shokoo et al., 2004;
Keyvanshokooh et al., 2007).
Sexual dimorphism
None found in morphometric and meristic characters although females
are said to be longer and heavier than males of the same age.
Colour
The back is ash-grey, blue-grey to greenish or dark brown, sometimes black, fading to a
white or cream belly. The contrast between the dark back and lighter rest of the
body is marked. Young often have a metallic sheen which fades with age. The snout is yellowish.
Size
Attained weights of 1228 kg yielding 246 kg of caviar or 7.7
million eggs (Berg, 1948-1949), even 1600 kg (Farid-Pak, no date), and
there are newspaper and other reports of fish 1200 kg and 6 m (Ottawa
Citizen 14 May 1986) or even 3200 kg and 9 m but such large fish are
not seen today and the largest sizes are probably exaggerations.
Modern catches are mostly much smaller than these exceptionally large
fish. A recent record with the specimen preserved in the Astrakhan
Museum in Russia is given in Sternin and Doré (1993) for a fish from
the Volga River in 1989 weighing about 980 kg, 4.3 m long and yielding
about 110 kg of caviar (Iran News, 14 July 1998, gives 988 kg, 120 kg
of caviar and an age of 60 years, presumably the same fish). A
photograph of a 1908 capture at Astrakhan in Stein and Bain (1981)
shows a fish weighing about 400 lbs (181.4 kg) containing 200 lbs (90.7 kg) of caviar worth
more than $69,000 in 1981. Tsepkin and Sokolov (1971) give some examples of large fish from former Soviet
waters. Birstein et al. (1997) consider this species to be the largest freshwater fish.
The mean weight of Caspian Sea fish decreased from 110 kg in the
early 1970s to 57 kg in 1991 (De Meulenaer and Raymakers, 1996).
Up to 2.83 m and 450 kg generally in Iran (Azari Takami et al., 1980)
but see below for news reports. Belugas up to 960 kg tried to enter
the Atrak River in 1836 (Vladykov, 1964). The longest fil mahi caught
in Iranian waters is apparently one taken on 23 February 1989 by
Turkmen fishermen at Shilat-e Nahee 4 in Mazandaran (see Abzeeyan,
Tehran, July 1991, page 3). It had a fork length of 4.5 m, a total
weight of 725 kg and a caviar weight of 98.2 kg. This individual was
worth U.S.$140,000 (Abzeeyan, Tehran, November 1992, page 57).
The heaviest fish from Iran is one reported by Hossein Aimani at 3000 lbs
(1360.8 kg) from near Babol in 1973
(www.amarillonet.com/stories/120599/bus_LQ7659.shtml, downloaded 7 March 2000).
Mobayen (1968) gives the largest Iranian specimen as 4.2 m and 850 kg.
Anonymous (1991a) and Sternin and Doré (1993) cite a
fish of 1742 lb (= about 791 kg), 7.5 feet long (= about 2.3 m) and
yielding 220 lb (= about 100 kg) of caviar from Iran in 1989, the
largest caught for 20 years; this may be the same fish as the previous
one as confusion in weights and lengths are common in reports of large
fishes. Other large specimens were taken at Mahmudabad, Mazandaran on
28 October 1992, measuring 3.2 m, weighing 430 kg and with 61.2 kg of
caviar (Abzeeyan, Tehran, November 1992, page 13), at Bandar-e
Torkeman (= Bandar-e Shah) weighing 320 and 410 kg giving 110 kg of
caviar for the two fish (Abzeeyan, Tehran 4(1):IIX, 1993), at
Bandar-e Torkeman, Mazandaran on 27 March 1993, measuring 4.0 m,
weighing 550 kg and with 81 kg of caviar (Abzeeyan, Tehran,
4(2):47, 1993), and in Mazandaran one measuring 3.0 m fork length and
3.4 m total length, weighing 960 kg and yielding 62.5 kg of caviar (Iranian
Fisheries Research and Training Organization Newsletter, 5:8,
1994). Newspaper reports in 1996 listed a fish of 500 kg with 54 kg of
caviar worth $107,000 and a fish caught in October 1997 at Babol Sar
weighed 300 kg, measured 3 m in length and had 45.1 kg of caviar. In
1998, one fish 3.4 m long yielded 43 kg of caviar (Reuters), a fish
caught off Bandar-e Torkeman on 2 February measured 3.75 m, weighed
405 kg and yielded 50 kg of caviar (IRNA (Islamic Republic News
Agency), 3 February 1998), one caught off Bandar Anzali on 25 October
weighed 360 kg, was 3 m long and yielded 24 kg of caviar and
"meat" worth 3.6 million rials (IRNA, 26 October
1998), one caught off Nour, Mazandaran on 15 November measured 3.5 m,
weighed 450 kg, yielded 53 kg of caviar and was 30 years old (IRNA,
16 November 1998), and one caught off Kianshahr, Gilan weighed 290 kg,
was 3.5 m long and yielded 50.6 kg of caviar worth 100 million rials (IRNA,
24 November 1998). In 1999 newspaper reports included one caught off
Bandar Anzali weighing 155 kg, carrying 31 kg of caviar worth $12,400
(IRNA, 31 October 1999), one caught off Talesh weighing 120 kg
with 23.5 kg of caviar worth 150-200 million rials (IRNA, 5
December 1999), and one caught off Bandar-e Torkman weighing over 405
kg with over 52 kg of caviar worth 500 million rials (IRNA, 14
December 1999). One fish caught near Bandar Anzali in weighed 370 kg
and yielded 51 kg of caviar (IRNA, 28 October 2002).
Distribution
Found in the Adriatic, Black and Caspian seas and their drainages.
Derzhavin (1934) reported it from the Babol, Sorkh and Gorgan rivers
but it was rare in the Safid River, although reported up to Kisom and
quite abundant in the sea off its mouth. Nedoshivin and Iljin (1927) record this
species from 10 river mouths while A. stellatus and A. gueldenstaedtii
are reported from 18; the 10 river mouths are Yusufabad, Musachai, Hasan Kiadeh,
Dastak, Safid, Kasumabad, Chalkarud, Sardabrud, Chalus and Kheirud. Kozhin (1957), Rostami (1961) and Armantrout
(1980) stated that it enters the Astara, Safid, Babol and Gorgan
rivers and the Anzali and Gorgan mordabs. It comprised only 0.5% in
numbers and 2.5% in weight of the Safid River catch in 1914-1915
(Nedoshivin and Iljin, 1927). Large numbers were caught in the sea off
Gasan-kuli in Turkmenistan near the Iranian border (Berg, 1948-1949).
Also reported from Hasan Kiadeh by V. D. Vladykov based on field work
notes made in 1962. More recently reported from the Gorgan and
Safid rivers, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea by Kiabi et al. (1999) and Abdoli and Naderi
(2009), from
the Safid River by Abbasi et al. (1999) and from the Safid, Gorgan and
Tedjen rivers. This species was not caught in a survey along the Iranian coast
in 2001 (Ivanov and Katunin, 2001). In 2004 there were plans to introduce this species to isolated,
natural waters bodies in Fars Province (H. R. Esmaeili, in litt., 2004).
Zoogeography
Presumably a relict of past isolation of the Black-Caspian seas from the world ocean.
Habitat
This sturgeon is found in large concentrations in the eastern
coastal region of the south Caspian Sea in all seasons. It is rare in
trawl catches, possibly because it has a more pelagic life than other
sturgeons. Fil mahi descend to greater depths than other sturgeons,
100-140 m in the Caspian and to 180 m in the Black Sea. There is no
seasonal variation in depth distribution in the south Caspian Sea in
contrast to the middle Caspian (Legeza, 1972; 1973). Only the young
are found in shallow, warm areas. On the spawning migration, this
sturgeon usually follows the deepest part of the river.
Most of this sturgeon's life is spent in the sea and it ascends
rivers only to spawn. The new-born sturgeon returns to the sea. Farabi et al.
(2007) examined salinity tolerance and physiology of juvenile fish in Iran. Only
the youngest fish showed mortality on direct transfer from fresh to estuarine
and Caspian sea water. Adults are typically found on silty or muddy bottoms in the sea but may be
found on shelly and coarse sand at a temperature range of 5.6-29.3°C
and depths of 5-140 m. In the southeastern Caspian it remains below 30
m in winter, entering shallower water at depths of 10-20 m in spring
as the temperature ameliorates, dispersing throughout the southeastern
Caspian in summer and migrating into Iranian waters in autumn (Legeza,
1972; Filippov, 1976). Depth distribution depends in large part on the
available food supplies.
Oxygen requirements are high, averaging about 14 mg/l, but they can
survive at 2-3 mg/l. Salinities up to 22‰ are tolerated. Feeding
occurs over a temperature range of 0.5-30°C and the spawning migration at a range of 6-21°C.
The highest densities in the southern Caspian Sea occur at 22-29ºC,
feeding in winter at 10-12ºC (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
Males become sexually mature at age 9-16 years and females at
12-22 years, varying with the spawning river. This is a very late maturation age among fishes
world-wide. Spawning intervals are 4-7 years for males and 5-7 years for females
(Vecsei et al., 2002; see below for other ranges but certainly intervals
are long for a fish species). Spring-spawning females (see below) first spawn at 201-209
cm, 50-60 kg and 17 years. Winter-spawning females first spawn at
181-190 cm, 30-39 kg and 16 years. Most spring females are 230-300 cm
long, weigh 80-160 kg and are 23-28 years of age. Most winter females
are 201-300 cm, 50-160 kg and 17-26 years (Raspopov and Dubinin,
1990). Spawning populations have a complex age structure, the Volga
River in 1936 had 50 age groups for example but only 28 in 1964. There
has been a trend for spawners to be younger. Average catches in former
Soviet waters of the Caspian Sea now weigh only 77 pounds (34.9 kg) each, a
decline caused by overfishing (Los Angeles Times, Part A, page
1, 28 August 1993). A life span of 150 years was reputed for this species but the greatest known
age for a Caspian fish is 75 years (Berg, 1948-1949). Most Caspian fish are now less than 20 years old and made
up of individuals from re-stocking programmes (De Meulenaer and
Raymakers, 1996). Raspopov (1993a; 1993b) gives the life cycle of
Volga River fish as 56 years, although this is not the maximum age. Kura River
sturgeon grow more slowly and mature later than sturgeon from the
Volga River. Growth in this species is rapid with 1-year-old fish in
the Caspian being 51 cm long and weighing 571 g. Growth is slower in
the Caspian than the Black Sea because of the decrease in numbers of Alosa
spp., the prime food item. Growth is also slower in the south Caspian than the
north (Caspian Sea Biodiversity Database, www.caspianenvironment.org). Hedayatai
et al. (2009) were able to correlate weight and length with immature male
gonadal stage, but not for females, in work directed to reducing maturation
time. Moghim et al. (2008) studied sex ratio along the Iranian coast for
the years 1990-2003 and found females dominated at 60-80% of landed fish.
Immature females decreased from 71 to 47% of the catch.
Levin (1997) summarises the spawning population of the Volga River
over the last 10 years as follows although he notes this population is
almost extinct. Rarely spawners enter between August and October and
breed after a winter hibernation. Other fish enter from December to
May with a peak from February to March. Peak spawning is in May with a
downstream migration to the Caspian Sea from June to September.
Females, comprising 20-24% of the spawning population, average 236-261
cm and 106-160 kg and are 17-21 years old with fish larger than 400 cm
being very rare. Males are 199-204 cm and 48-55 kg and are 11-18 years
old. Spawning occurs at 9-11°C.
Farid-Pak (no date) gives approximate weights for Iranian beluga of
75-100 kg and 2.0-2.5 m, and a yield of 17-20 kg of caviar per female.
2608 beluga from Astara in Azerbaijan averaged 168 cm for males and
192 cm for females.
Von Bertalanffy growth parameters in Iranian females are L∞
= 320 cm and K = 0.065 for juveniles, 450 cm and 0.029 for the middle
stanza and 533 cm and 0.023 for older fish and for males 270 cm and
0.086 or 302 cm and 0.072, depending on the methodology used. Total
mortality (Z) was 0.21-0.67 for females and 0.22-0.75 for males,
natural mortality (M) was 0.03 for females and 0.05 for males, fishing
mortality (F) was 0.45 for females and 0.33 for males, and optimum
fishing mortality was (F) 0.07 for females and 0.16 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
Taghavi Motlagh (2001) gives more complete data (on which the previous summary
was based) on growth, mortality and yield-per-recruit on this species from 1995
to 1999 in the Iranian Caspian Sea. He concluded that fishing mortality should
be stopped. Maximum age in his sample was 46 years.
Food
In contrast to other sturgeons, this species is a pelagic predator as adults.
Even sea birds and seals may be eaten. However, the introduced polychaete worm Nereis is now a mainstay of
the diet of this species in the north Caspian Sea. Other foods are
molluscs, formerly a main food, and small fish such as Rutilus
rutilus (and presumably R. caspicus)and gobies (Gobiidae). Fish are the main diet item when
large, invertebrates when young. This species needs to find thick concentrations
of small or large fishes in order to feed actively; in the north Caspian these
are kilka and fish on migration at fishways and in the midde Caspian spawning
atherinids and commercial herrings (Polyanina et al., 1999). The fish found by Azari Takami et
al. (1980) in Iran were gobies, Cyprinus carpio, Liza,
and Rutilus. Gobies are a favourite food item but bivalves and
crustaceans are taken if fish are absent. Filippov (1976) notes that
large specimens eat sturgeons such as sevryuga, kopur (Cyprinus
carpio), mullets (Mugilidae), birds such as coots, and baby seals
and because of its pelagic life takes the clupeids Alosa
braschnikowii and Clupeonella caspia and also the
shrimp Leander adspersus. Crabs are also eaten. The principal
food as percent by weight in the southeastern Caspian was Neogobius
fluviatilis (= pallasi) (up to 78.1%), gobies accounted for up to 81.2% and
fish 81.6-100%. Crustaceans accounted for up to 7.8% and molluscs only
up to 0.2%. The cyprinid, Chalcalburnus (= Alburnus) chalcoides, is also
eaten (Mageramov and Zarbalieva, 1989).
Reproduction
Roux (1961a) maintained that this species did not reproduce in
Iranian rivers but Rudin (1966) said that they inhabited the Safid and
Gorgan rivers. The main spawning river was the Volga as 90% of the
Caspian stock reproduced there, travelling as far up as the Moskva
River. Males arrive at spawning sites before females. Despite their
size, these sturgeons may leap out of the water on the spawning run
and possibly during spawning. Adhesive eggs are deposited on sandy
substrates, with rocky and gravelly bottoms near the bank, in the
strong current of mid-river (1.5-2.0 m/sec.). Water temperatures are 9-17°C
and eggs develop in 9-10 days (Novikova, 1994; Vecsei et al., 2002). Spawning usually takes
place at a depth of 4-15 m, sometimes as deep as 40 m. Weight loss
after spawning may reach 50% and females are only ready to spawn again
after 5-6 years and males after 3-4 years (4-8 and 4-7 years in Speer et al.,
2000). The migration in the Volga River occurs year-round with peaks in spring (<30% of the stock)
and autumn. The spring race reach the spawning beds in the same year,
reproduce and return to the sea. The winter race, migrating in summer
and fall, overwinter in the river and reproduce the following spring.
The spring run is in March and April and the winter run in September
and October. The chief spawning period in the Kura River is from the
end of May to the beginning of June (Zakharyan, 1972) and fish were
found as far up as Tbilisi (= Tiflis).
Fecundity reaches, exceptionally, 7,729,700 eggs but does not
increase with age for fish of equal length and weight (Raspopov, 1987;
Raspopov and Dubinin, 1990). Mean fecundity for the Volga stock was
531,600 eggs. Normal deposition of eggs is 500/sq m in the Volga but
densities fell below 5/sq m in the 1980s, as low as 0.2/sq m and with
an average of 1.5/sq m (Novikova, 1994). Kura River sturgeon are less
fecund than Volga sturgeon. Egg diameter reaches 4.3 mm. Eggs are a
dark silver and oval. Larvae hatch in 10-14 days, the yolk sac is absorbed in
10-14 days and feeding larvae move downstream at up to 60 km/day (Vecsei et al., 2002).
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) describe a new species of acanthocephalan, Corynosoma
caspicum, and also Leptorhynchoides plagicephalus from this
sturgeon in Iran. Mokhayer and Anwar (1973) report on sturgeon
parasites in general (see under Acipenser gueldenstaedtii).
Mokhayer (1976b) reports gas bubble disease in Iranian sturgeons
without specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Pourgholam (1994) reports the coelenterate Polypodium hydriforme
from this species caught on the Babol Sar and Bandar-e Torkeman
fishing grounds in Mazandaran. Larvae of the nematode Anisakis
simplex and the acanthocephalan Corynosoma strumosum are
also reported from this species (Annual Bulletin 1993-94, Iranian
Fisheries Research and Training Organization, Tehran, p. 48-49,
1995). Sattari et al. (2002) record Cucullanus sphaerocephalus,
Eustrongylides excisus, Skrjabinopsolus semiarmatus, Anisakis
sp., Eubothrium acipenserinum and Corynosoma strumosum, the fauna
being similar to other sturgeons because of their piscivorous feeding. Gorogi (2006b) recorded the nematodes Cucullanus sphaerocephalus and
Anisakis schupakovi, the cestode Eubothrium acipsenserinum and the
acanthocephalans Leptorhynchoides plagicephalus and Corynosoma
strumosum from Iranian waters. Sattari and Mokhayer (2005a; 2005b) recorded
the occurrence of parasites in this species from the Iranian southwestern and
central coast of the Caspian Sea. The species found were the nematodes
Cucullanus sphaerocephalus, Eustrongyloides excisus and Anisakis
sp., the cestode Eubothrium acipenserinum, the acanthocephalan
Corynosoma strumosum, the digenean trematode Skrjabinopsolus semiarmatus.
General conclusions were that the diversity of parasites was less in Iranian
waters than in the northern Caspian Sea, perhaps a reflection of the more varied
habitat, its productivity and the carbonate ions differing between the two
regions. The diversity of parasite seems to have declined over time also,
perhaps as a result of unfavourable environmental conditions, particularly in
the freshwater ecosystem which limits the waters available for spawning and parasite acquisition.
Shenavar Masouleh et al. (2006) found hatchery fingerlings to harbour
Diplostomum spathaceum and Trichodina sp.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
The fil mahi is so large that its predators are only effective on
young fish. They include Sander lucioperca and Silurus
glanis and, needless to say at all sizes, mankind.
Economic importance
This species provides the best caviar according to Borodin (1930).
The large eggs fetch a higher price on the American market. Up to 80% (3000 kg
in 2002) of the legal beluga caviar export is consumed in the U.S.A. (Hamilton,
2002). A 1227 kg specimen caught in Russian waters in 1924 gave 245 kg of caviar worth
£189,350. In the 1990s, a 225 kg fil mahi could yield 22 kg of caviar
worth $120,000 (Trickey, 1995). Catches in the Volga region in the
1970s were in the range 740-2650 tonnes and in the 1980s 460-900
t comprising 4.4-12.2% and 3.7-4.4% respectively by weight of the
total catch of all sturgeons there. The highest catch in the Caspian
Sea was in 1902-1907 (Birstein, 1993). Khodorevskaya et al.
(1997) and Khodorevskaya (1999) summarise the decline in catches and
make the startling observation that 96.3% of all fil mahi in the Volga
River are hatchery reared.
Fil mahi were fished intensively off the Iranian coast in the
southeastern Caspian and in 1950 amounted to 38.6% of the total
sturgeon catch. During the five-year period 1957/1958 to 1961/1962 fil
mahi catches in the Gorgan Division of the Iranian fishery varied
between 86-90% of total Iranian catches. The Atrak River estuary area
was particularly important for this species. Catches of the oldest age
groups has declined and the proportion of young and immature fish has
increased. Iranian rivers suitable for this sturgeon were the Safid
and the Gorgan but both are now regulated so Iranian stocks are
probably maintained by fish reproducing in the rivers of the former
U.S.S.R. (Filippov, 1976). Fil mahi cannot be managed by Iranian
authorities therefore. However the "Gharasoo" Research
Station in Mazandaran is researching the culture and release of fil
mahi up to 1 kg (Madbaygi, 1993b) and farming through pen culture in
Gorgan Bay (Iranian Fisheries Research and Training Organization
Newsletter, 11:6, 1996). Two million "roes" (presumably
young fish) were released into the Caspian Sea from Mazandaran prior
to 1 June 1995 with a further 2 million to be released later in the
year (http://netiran.com/news/IRNA/html/950701IRGG08.html). In 1997,
852 fishermen were fishing for fil mahi on the northern Iranian coast
(Anonymous, 1997c).
Farid-Pak (no date) gives the months of September-October and
March-April as the most important for the fisheries of this species.
Nevraev (1929) gives catch ranges of 109-3100 fil mahi individuals for
the Astara region of Iran over the period from 1901-1902 to 1913-1914,
for the Safid Rud region 104 to 730 individuals for the period
1899-1900 to 1913-1914, for the Mazandaran region 31 to 491
individuals for 1906-1907 to 1913-1914, and for the Astrabad (=
Gorgan) region 688 to 1764 individuals for 1902-1903 to 1913-1914.
Vladykov (1964) records average yearly catches in Iran of this species
from 1927/28-1931/32 to 1957/58-1961/62 with ranges of 57,820-418,059
kg body weight (5.4-33.0% of the total sturgeon catch) and 2038-32,873
kg caviar (2.6-20.4%). There was an upward trend in caviar production
from this species in the 1950s (Vladykov, 1964). RaLonde and Walczak
(1970b) summarise yields for the years 1963 to 1967 in Iran of meat
and caviar as 572.3 tonnes (40.1 tonnes), 583.5 (47.3), 575.8 (39.1),
458.1 (29.5), and 507.2 (30.0) respectively. A commercial house
maintains (1995) that caviar from this species comprises only 3% of
the total catch. Taghavi Motlagh (2001) noted a decline in the share of Iranian
caviar production from 18% in 1971 to 4% in 2000.
This species has been studied in ponds as breeders are used to produce fingerlings which are then
available as experimental fish for chemical and growth studies. Ghorbani et
al. (2003) studied the influence of heavy metals on the level of alfa-amylase
activity in the digestive tract and found decrease in enzyme activity was not
significant. Karimzadeh et
al. (2005) studied cytochrome P4501A1, a major isoenzyme in the
monooxygenase system which can be induced by polycyclic aromatic hydrocarbon
pollutants. Khoshbavar Rostami et al. (2006) studied the effects of
polyaromatic hydrocarbons from Caspian Sea oil wells on 8.5 g fingerlings and
found these chemicals to seriously affect the fish blood and enzyme systems.
Khoshbavar Rostami et al. (2004; 2006) studied the
organophosphate diazinon and its deleterious effects on haematological
parameters in this sturgeon. Sharifpour et al. (2004) studied the effects
of the insecticide endosulfan, sturgeon weighing 3-5 g showing irregular
swimming, whirling, convulsions, with other conditions, and eventually death.
Endosulfan is highly toxic to beluga fingerlings. Sudagar et al. (20050
examined the addition of betaine and methionine (an important nutrient and an
enzyme) to the diet of juvenile beluga. The fish showed improved weight gain,
weight gain percentage, specific growth rate, protein efficiency ratio, net
protein utilisation, condition factor, survival, and price index at enrichment
levels of 0.5% betaine and 1% methionine. Ghorbani et al. (2004) examined
the influence of a series of microelements (zinc, nickel, cobalt, manganese,
iron and copper) on the level of proteolytic enzymes and alkaline phosphatase
activity (used for enzyme inmmunoassays) in the digestive tract of juvenile
beluga. Most treatments showed the level of enzyme activity was less than the
control. Shahsavani (2002) determined blood parameters of fingerlings from fish
farms and found the fish to be healthy. Blood parameters are used to indicate
physiological condition and sublethal stress due to endogenous and exogenous
changes, hence the need to determine normal values. Askarian et al.
(2006) looked at serum osmoregulatory parameters under different light regimes,
one form of physical stressor in aquaculture of this endangered species. No
differences in serum cortisol levels were found between treatments although
elevations of serum cortisol, glucose and triglyceride occurred in a continuous
dark regime. Gafarian et al. (2007) used probiotic bacillus in the
feeding of larval sturgeon and found that it positively affected feeding
efficiency and levels of carcass nutrient composition.
Ebrahimi (2006) determined that the earliest time to transfer farmed larvae to a
commercial diet from a natural one was 3-4 days after yolk sac absorption and
the best time was 30 days at an average weight of 2 g.
Khoshbavar-Rostami et al. (2007) examined the immune response to
Aeromonas hydrophila bacterin.
Soulati and Falahatkar (2007) looked at stress response in sub-yearlings exposed
to air. Shamloufar et al. (2007) examined the sub-lethal effects of
diazinon on haematological indices in juveniles. Akrami et al. (2008)
studied the effect of prebiotic inulin levels and found it did not increase
growth performance of juveniles. Askarian et al. (2008) examined the gastrointestinal tract for
lactic acid bacteria and found the population levels to be significantly higher
than in Acipenser persicus. Bagheri et al. (2008) studied seasonal
hormone fluctuation in fish raised in saline conditions at Bafgh, Yazd Province. Hedayati et al. (2008) studied blood
indices of fish cultured in brackish water. Hosseini et al. (2008) examined the
organochlorine content of four sturgeon species and found fil mahi had four
times more than the next highest species (A. nudiventris); generally
pollutants had been reduced compared to previous studies but some specimens
exceeded guideline levels for food. Soltani et al. (2008) found that
100-200 mg/kg of vitamin C was optimum for rearing this sturgeon. Baghfalaki
et al. (2009) carried out studies on seminal plasma indices in order to
improve short and long-term storage of semen. Darvish
Bastami et al. (2009) found that addition of Daphnia and Artemia extracts
had positive outcomes on growth in juveniles. Akbari et al. (2009)
studied the use of sperm extenders and found that they prolonged spermatozoa
viability in short-term storage and prolonged sperm motility. Ghanbari et al.
(2009) isolated Lactobacillus species, which ferment carbohydrates, from
the intestine. Jalali et al.
(2009) found that Artemia urmiana nauplii on enriched with HUFA and
vitamin C and fed to larval sturgeon improves some growth and stress tolerance. Seifzadeh et al. (2009) examined microbial quality of packaged fillets of
this sturgeon.
Askarian and Kousha (2008) examined food ration on the acute stress response,
those receiving a high ration performing better. Alizadeh et al.
(2009) studied effects of different diets on energy levels and gonad development
for fish reared in inland brackish water, this environment proving suitable. Askarian and Kousha
(2009) studied photoperiod in rearing year-old fil mahi evaluated by growth (no
effect) and serum parameters (various individual responses to stress. Falahatkar
et al. (2009) examined dosages of vitamin C that enhanced immune
responses to disease. Jalali et al. (2009) found that juveniles fed
Ergosan, an algal product, had higher growth rates than a control group and
certain haematological parameters were affected. Sepahdari et al. (2009) found various skin lesions
in fish fed a diet containing aflatoxin B1 (a naturally occurring
fungal toxin). Gharaei and Ghaffari (2010) found that serum biochemistry can be
used as enzyme biomarkers to assess toxicity of methyl mercury in juveniles.
Gharaei et al. (2010) studied accumulation of methylmercury in different
tissues of hatchery juveniles fed four different concentrations of MeHg. Sepahdari et al. (2010) found deleterious changes in liver
tissues
n fish fed a diet containing aflatoxin B1. Yousefi et al.
(2010) found nucleotides added to the diet of juveniles had a positive effect on
stress tolerance. Alizadeh et al.
(2011) studied seasonal changes of blood serum ions in brackish water,
a proposed culture medium,
at Bafgh in Yazd Province. Ebrahimi Dorcheh
and Zare (2011)
found that the optimum dietary level of lipid for fingerlings was 14%. Filizadeh and Rajabi Islami (2011) reported on on the
toxicity of the herbicide glyphosate. Ghanbari and Jami
(2011b) recorded Lactobacillus species from the guts of this species.
Peik Mousavi et al. (2011) determined the role of methionine in whole
body composition of juveniles. Rafatnezhad et al. (2011) stocked juveniles in fibreglass tanks at
differing densities and found that water quality varied significantly and, as a
result, growth. Ta'ati et al. (2011) found that growth performance and some
immunophysiological indices improved when juveniles were fed diets containing
the commercial prebiotic Immunoster (a brewers yeast cell wall derivative).
Agh et al.
(2012) examined first feeding strategies for hatchery larvae finding live food
and manufactured diet improves weight gain, although pure live food increased
survival. Yarmohammadi et
al. (2011)
found sex chromosomes were not differentiated sufficiently using AFLP to
identify males and females at an early life stage. Jahanbakhshi et al.
(2012) found that the insecticide cypermethrin to be highly toxic to juveniles.
Jahanbakhshi et
al. (2012) examined the use of plant protein to replace fish meal in feed
for juveniles in hatcheries. Karimzadeh et al. (2012) used cytochrome
P450 1A in this sturgeon as a biomarker for impact assessment of polycyclic
hydrocarbons pollutants. Mohseni et al. (2012) investigated the best
live food (Daphnia, chironomids, gammarids) and formulated diet in
various combinations for larval fish and found chironomids plus formulated diet
was best, other diets showing frequent cannibalism and higher mortality.
Poursaeid et al. (2012) examined haemtological and cortisol values of
female fish implanted with cortisol (to imitate chronic stress) after an
endoscopic examination to assess gonad development. Falahatkar et al.
(2013) evaluated the winter feeding rate in young-of-the-year and its effects on
maintaining weight during short periods of winter starvation (1% body weight per
day for commercial farming and 0.3% in overwintering ponds).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria,
as food and in textbooks.
Conservation
See also under A. gueldenstaedtii. Critically endangered in Turkey
(Fricke et al., 2007). Despite loss of 99% of
the Volga River spawning beds to dam construction, natural
reproduction increased over a recent five-year period, but continues
to be dependent on the variable flow-regime (Raspopov and Dubinin,
1990). Novikova (1994) estimated the capacity of the Volga spawning
grounds to be 9-11,000 fish. A major problem in the 1990s was
poaching. Trickey (1995), referring to Russian stocks, expected a
legal harvest of 4400 tonnes with poachers taking twice that amount.
This legal and illegal catch is still less than catches of 20 years
ago, primarily because of pollution. Birstein (1996) records the catch
of the Volga delta hatcheries in 1995 to be only 35 fish, insufficient
for artificial reproduction. Natural spawners are taken by poachers.
The level of poaching in the Ural River is also high, and this was the
only river where some natural reproduction was going on. The fil mahi
has effectively stopped reproducing in the Caspian Sea.
Moghim et al. (no date) note that juveniles of this species are caught
in the beach seine fishery for other species in Mazandaran. During 2001-2002,
23,760 seine hauls had a by-catch of 6% for this species among sturgeons
captured.
Khodorevskaya and Novikova (1995) point out that cooperation among
all the Caspian Sea states is needed to maintain this species along
with an annual release of at least 20 million young from hatcheries.
Fingerlings released per year from 1998 to 2002 range from 6.9 to 12.6 million
for all Caspian states (CITES website). Spawning migrations are now seen only in the Volga and Ural rivers,
the Kura, Terek and Sulak rivers no longer supporting stocks. The
Volga migration was 25,500 fish weighing 2600 t in the early
1970s but has fallen to 11,700 fish weighing 750 t. The
commercial catch fell from 2000 t to 500 t. In the Volga
River 96.3% of the spawning population consists of hatchery fish
although the Ural River maintains a naturally reproducing stock.
Since stocks are maintained mostly by artificial rearing, this
sturgeon has been proposed for inclusion in the "Red Book of the
U.S.S.R." which forms the basis for measures to protect species
(Pavlov et al., 1985; Mina, 1992). Stocks have been increased
through rearing and natural reproduction in the Ural River, the number
rising from 9.6 million in 1976 to 15.3 million in 1983, so the status
of this species was then regarded as acceptable. However Lelek (1987)
and Birstein (1993) list this species as vulnerable to endangered.
Kiabi et al. (1999) consider this species to be endangered in
the south Caspian Sea basin according to IUCN criteria as does IUCN and CITES (Vecsei
et al., 2002). The U. S. Fish and Wildlife Service lists it as threatened
under the U.S. Endangered Species Act as of 21 October 2004 (http://news/fws.gov/newsreleases,
(dated 20 April 2004) and downloaded 22 April 2004) and the Wildlife Service
has been petitioned to make it endangered (Speer et al., 2000).
Endangered status would stop importation of flesh and caviar to the United
States. Suspension of trade in this species from the Black Sea basin by
the U.S. Fish and Wildlife Service was instituted in 2005 (Federal Register,
2005) and imports from Iran are banned for political reasons along with other
sturgeons. Criteria for the various status assessments
include commercial overfishing (fishermen cannot even catch the set quotas),
failure of regulatory oversight, few in numbers, habitat destruction, dams
preventing spawning migrations, medium range (25-75% of water bodies), absent in other water bodies in Iran, poaching,
pollution, diseases due to pollution, and presence outside the Caspian Sea basin. The World Wildlife
Federation (WWF) listed this species as number 4 on the top 10 most
endangered species in the world (www.extravalue.com/sturgeon.shtml,
downloaded 13 March 2000). The species status may be changed to Appendix I on
the CITES listing, when international trade in its caviar would be banned (Vecsei
et al., 2002). The export quota for this sturgeon in the Caspian Sea 2004 was reduced to 4425
kg although an illegal harvest was still substantial (www.tehrantimes.com, downloaded 14 October 2004).
Illegal fishing from 1990 onward and cessation of hatchery releases
will lead to loss of the stock unless an agreement between Caspian
states can be reached to protect this species.
The invasion of the ctenophore Mnemiopsis has led to declines in the
kilka (Clupeonella spp.) stocks, a prime food of fil mahi (Kideys, 2002).
Caviar from Russian caught fil mahi bought in New York stores has
been examined for pollutant content (Boyle, 1994). Three stores
carried caviar with 3.17-3.27 parts per million of DDT plus its
metabolites DDD and DDE, 410-640 parts per billion of the PCB Arclor
1254, and 2.1-2.8 parts per million selenium. These values are below
the U.S. Food and Drug Administration's action levels of 5 parts per
million for DDT, 2 parts per million of PCB and 10-50 parts per
billion of selenium in drinking water. Nevertheless they are cause for concern.
Various studies have been carried out on the aquaculture of this valuable
sturgeon in Iran. Mohseni et al., (2000) have studied effective stocking density of eggs and
larvae in incubators and rearing tanks in order to maximise production and avoid
various morphological deformities.
Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
32 |
29 |
29 |
48 |
16 |
| Injected broodstock |
19 |
14 |
21 |
30 |
9 |
| Spawning rate * (%) |
74 |
71.4 |
62 |
65 |
77 |
| Fertilisation rate (%) |
55 |
65.5 |
65 |
54 |
65 |
| Survival rate in incubators
(%) |
62 |
73.4 |
62 |
32 |
72 |
| Survival rate in tanks (%) |
80 |
62 |
56 |
100 |
79 |
| Stocking density in ponds
(fish/ha) |
82,100 |
51,639 |
51,333 |
52,359 |
65,448 |
| Survival rate in ponds (%) |
73 |
51.3 |
67 |
43 |
59 |
| Fingerling production (x
1000) |
1900 |
640 |
24,037 |
42 |
146 |
* Rate of response to hormone injection
Mohseni et al. (2006) studied the best stocking density for rearing juveniles less than one
year old weighing 92.09 g on average and one-year-old fish weighing 918.14 g on
average. Stocking densities were 1.6, 2.8 and 4.0 kg/m2 for the
juveniles and 1.5, 2.5, 3.5 and 4.5 kg/m2 for the older fish.
Increased density had a negative impact on growth, body weight, specific growth
rate and food conversion ratio in both experiments. Higher concentrations
of fishes even had malformed caudal fins and body injuries from increased
contact. Recommended stocking densities were 1.5-2.0 kg/m2 for fish
up to 90 g and 2.5-3.0 kg/m2 for fish over 900 g.
Cage culture of fingerlings has been carried out in Gorgan Bay
starting in 1992. Cages were 3200 sq m with a depth of 2.5 m and
contained 11,500 fingerlings. Over 16-17 months average weight
increased from 20 g to 1365.5 g, to a maximum of 2200 g. Mean fork
length was 58.6 cm. Food in the first phase was a concentrate of
ground carp and kilka but in later phases natural foods such as
benthos and fry were used. The preliminary results indicate economic
feasibility for cage culture (Iranian Fisheries Research and
Training Organization Newsletter, 7:4-5, 1995; Annual Bulletin
1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 46-47, 1995).
Kamali and Farabi (2005) showed that juveniles weighing
20 g or more adapted better to concentrated feed in fibreglass tanks. Mohseni
et al. (2004) studying growth rate, food conversion ratio and survival in
fingerlings held in fibreglass tanks found these factors to be dependent on
higher feeding frequencies (3, 5 and 8 times per day). Akrami et al.
(2005) found Cladocera were the primary prey of fingerlings in earthen ponds
with chironomid larvae and ostracods secondary prey, and the copepod Cyclops
an occasional prey. Condition factor and growth decreased as weight and length
of fingerlings increased. Growth was was positively allometric (b>3). Mohseni et al.
(2005) found growth of fil mahi was better in fibreglass tanks but later in the
rearing process the trend reversed and earthen tanks showed a better condition.
Mohseni et al. (2006) examined the effects of feeding rates (1, 2, 3 and
4% of biomass) on various factors for fish weighing an average 867.9 g and fed
for 100 days in fibreglass tanks. Increase in feeding ratio directly increased
daily food consumption and negatively affected the feeding efficiency, food
conversion ratio, specific growth rate and price index. When fish were given 2%
of the body weight, one unit of meat was produced from 1.92 units of food. A
second trial with feeding rates 0.75, 1.5, 2.5 and 3% took place with fish
weighing 2096.1 g and fed for 125 days. Feeding with 0.75% produced one unit of
meat per 1.82 units of food consumed. Fatemeh and Armin (2005) studied the
effect of photoperiod on growth in one-year-old fil mahi. Extended day length
had a positive effect on growth rate, specific growth rate, weight and length,
and condition factor. The organophosphate diazinon was studied experimentally by
Khoshbavar Rostami et al. (2006) as to its effects on haematological and
biochemical factors of the blood serum of this fish. Falahatkar et al.
(2006) experimented with various levels of vitamin C as a diet supplement and
recommended 200 mg kg-1 during the first weeks of growth and development.
Mohsen et al. (2008) found that diets supplemented with L-carnitine
improved growth rate, feed utilisation and stimulated protein-sparing effect. L-carnitine
is a vitamin-like compound found naturally in fishes and is involved
transporting long-chain fatty acids in metabolism. Ahmadifar et al.
(2009) found that dietary Ergosan had some positive effects on growth and
haematological parameters (Ergosan comprises algines and
polysaccharides known to strengthen the full range of natural defence systems in
fish).
Nezami et al. (2000) maintain that despite artificial spawning and fingerling
production, restoration of this species in Iran was not very successful.
Abdolhay et al. (2006) report on 17 adults caught in 1998 of which 10
fish were injected with hypophysis extract and produced 1.08 million fingerlings
while in 2002, 29 were caught and 21 produced 2.4 million fingerlings. Azari Takami (1999) cites production of 300-350 kg/ha in 40
days with 106,000 fingerlings produced per 15 females in 40 days with a release
weight of 10-15 g. Spawning fish were captured in the sea as they no longer
migrated into Iranian rivers and propagation results were not as good as in
previous years (420-587 kg/ha in 25 days, 690,000 per 2 females, release weight
5-8 g). About 1 million fingerlings were released into the Caspian Sea.
Iranian releases of fingerlings were 687,400 (1988), 406,100 (1999), 1,900,919
(2000), 700,000 (2001), 2,403,794 (2002) with ca. 4 million proposed for 2003
(CITES website). The annual release of fingerlings weighing 3-5 g into the Caspian from Iran is
1-2 million fish and some of these are tagged for future studies (Iranian
Fisheries Research Organization Newsletter, 39:1, 2004). In 2001, 8 females and 12-14 males were caught in Gilan, about
half of which could be used as broodstock at the Shahid Beheshti hatchery (Raymakers, 2002).
Fingerlings have been raised in fibreglass ponds in brackish and fresh waters
in Iran (Iranian Fisheries Research Organization Newsletter, 35:3, 2003;
H. Pouralifashtomi in the 5th International Symposium on Sturgeon, Iranian Fisheries Research
Organization, 9-13 May 2005, Ramsar; Pouralifashtomi, 2006).
Growth was better in brackish water when fed diets containing 45% protein and
12.8% fat. Studies of cultured male fil mahi show that
they attain maturity at 8-10 years, earlier than fish in natural habitats,
indicative of their potential for caviar production under culture conditions (Iranian
Fisheries Research Organization Newsletter, 39:3, 2004).
Cultivation of this species in earthen ponds in the central
Iranian desert at Bafqh near Yazd has been carried out. After three months at 24ºC
and a salinity of 12.5‰ the fish reached 250 g with a survival rate of
60%, after six months at 16ºC and 11.0‰ the fish weighed 1100 g with a survival rate of 96%. Growth was better during
the cold season (Iranian Fisheries Research Organization Newsletter, 34:3; 36:4, 2003).
Further work
See under A. gueldenstaedtii.
Sources
See under family above. Babushkin (1964) gives a general review of the biology and catch of this species.
Iranian material: None.
Comparative material: CMNFI 1986-0147, 1, ca. 305 mm total length, Romania, Black Sea at Sulina (45°09'N, 29°41'E).
Pseudoscaphirhynchus
Nikolskii, 1900
Pseudoscaphirhynchus kaufmanni
(Kessler, 1877)
This species is reported from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan by Shakirova and Sukhanova (1994) and
Sal'nikov (1995). It may eventually be found in the Tedzhen River and
Caspian Sea basins of Iran. No Iranian record.
Clupeidae
Herrings, shads, sardines, pilchards and menhadens are
moderate-sized fishes, usually less than 25 cm long, found in warmer
marine waters with some species anadromous or permanent freshwater
residents. There are about 56 genera and about 200 species world-wide (Nelson, 2006; Eschmeyer
and Fong, 2011), with 8 species in
the Caspian Sea and 1 commonly found in Persian Gulf drainages. Some other species are
known to enter rivers in southern Iran (see Marine List in Checklists in Introduction).
The diversity of this family in the Caspian Sea is seen in the number of subspecies which
have been described, rather than in genera. At the species level there are several endemics.
Curiously, the species and subspecies in the Caspian are generally
of larger size than their relatives in the Black Sea basin. These
observations are attributed to the variable environment in the Caspian
Sea over time, with repeated changes in salinity and temperature which
the fish could not avoid. Black, Mediterranean and Atlantic species
lived under more stable conditions and could, in any case, retreat
from lowered temperatures for example. In addition, the Caspian Sea
clupeids lacked the competitors which entered the Black Sea from the
Mediterranean and Atlantic and some (Clupeonella spp., Alosa
caspia) could occupy the pelagic, planktivore niche taken up by
other species in the Black Sea. There are no other pelagic fish but
these herrings in the stable salinity areas of the Caspian Sea.
These fishes usually have modified scales on the belly forming
abdominal scutes with a saw-like edge. Most species have 2, long,
rod-like postcleithra. The lateral line is usually absent or on only a
few scales. Silvery cycloid scales are easily detached and are found
only on the body. The mouth is usually terminal with jaws about equal
in length. Teeth are small or absent but gill rakers are long and
numerous for sieving plankton. Fins lack spines and there are no
barbels. There is no adipose fin. The pectoral and pelvic fins have a
large axillary scale. The caudal fin is deeply forked. The eye is
partly covered by an adipose eyelid. The flesh is particularly oily
and is highly nutritional.
Members of this family often form immense schools in surface waters
of the ocean and the Caspian Sea where they feed on plankton.
Schooling is an anti-predator device making it difficult for a
predator to pick out an individual from a tight mass of fish. There is
also a "sentry effect" where awareness is increased by the
presence of many fish. The school is maintained by a balance between
visual attraction and lateral line stimulus repulsion. Herring can
feed on the smaller plankton, less than 300-400 µm, at night by
filter-feeding but during the day can also use particulate feeding. In
the latter, they select larger plankton using the area temporalis, a
specialised ventro-posterior region of the retina which improves
vision as herring approach food items from slightly below.
Herring are easily caught and are extremely valuable to commercial
fisheries. They are the most important fishes economically, both as
food for man and also for many other commercial fish species. Wars
have been fought over fisheries for herrings. In one year, members of
the herring family made up 37.3% of all fish caught in the world. Some
are used for fish meal, as fertiliser and as an oil source. The
1994-1995 catch of clupeids in the Iranian Caspian was 98.3 tonnes by
beach seine and 671.5 t by gill nets, a decrease of 200 t in
total over the previous year's catch (Iranian Fisheries Research
and Training Organization Newsletter, 10:4-5, 1995)(but see later
under Clupeonella where catch is much higher). The Caspian Sea shads
account for about 35% of total inland production in Iran which was
117,300 t in 1995 (Bartley and Rana, 1998). These fish are used
in a high value form as frozen whole consumer packs, as fish meal for
poultry and in aquaculture, and in canning (Food and Agriculture
Organization, Fisheries Department, 1996).
The catch of "sprats" (Clupeidae) in Azerbaijani waters
is near extinction through poor fishery management according to Golub (1992).
Major sources for the biology and systematics of Caspian clupeids
remains Svetovidov (1952), now inevitably dated but not yet updated,
Whitehead (1985) and Hoestlandt (1991). There has been no recent,
careful systematic and taxonomic study of these species in the Caspian Sea basin
and extensive new material was not available for examination here.
Genus Alosa
Linck, 1790
The Caspian species of Alosa were formerly placed in the
genus Caspialosa Berg, 1915. Svetovidov (1952) synonymised the
genus Caspialosa Berg, 1915 with Alosa. There are 5
species in Iranian waters and the Caspian Sea as a whole but numerous
subspecies have been described. Alosa species are also found in
the Black Sea, Mediterranean Sea and Atlantic Ocean.
Often distinguished by gill raker counts which in any case overlap,
the various subspecies are difficult to identify. Morphometric
characters are of little help and Zamakhaev (1944) points out that
some named taxa are merely different age groups. This problem is
commented on further in the Species Accounts.
Caspialosa suworowi (Berg, 1913) (also spelt suvorovi
in the literature) has been used for hybrids of various Caspian
herrings and is not a valid species (Berg, 1948-1949). The holotype is
in the Zoological Institute, St. Petersburg under ZISP 15927 (Svetovidov,
1952; Eschmeyer et al., 1996).
Alosa species are distinguished from sympatric Clupeonella
species by larger size (up to 75 cm total length compared to 20 cm), a
large mouth, a black spot on the flank behind the operculum and
sometimes a row of such spots, an elongate scale or ala at the upper
and lower base of the caudal fin, a notch at the mid-line of the upper
jaw and by the last two anal fin rays not being elongated.
Caspian Sea species have a laterally compressed belly with 29-36
spiny scutes running from the throat to the anal fin; the dorsal fin
origin is closer to the snout tip than the caudal fin base; the dorsal
fin lies in a groove formed by enlarged scales; scales are easily
detached; the pelvic fin origin lies below or slightly posterior to
the dorsal fin origin; teeth are usually present on the jaws, roof of
the mouth (on the palatine bone and always on the vomer bone), and on the tongue;
the opercular bone is distinctly striated; eggs are demersal,
semi-pelagic, and lack an oil globule; gill rakers highly variable in
shape and number (18-180); dorsal fin branched rays 11-16, anal fin
branched rays 10-21, scales in lateral series 49-60, and vertebrae 43-55.
Afraei Bandpyi et al. (2004) examined Alosa species from
Mazandaran and Golestan provinces and found the following distinguishing
characters:-
| Species |
Gill rakers |
Ratio of eye diameter to total length (%) |
| A. braschnikowii |
20-40, mean 30.9 |
2.9-5.8, mean 4.7 |
| A. caspia |
110-125, mean 118.3 |
5.7-7.5, mean 6.2 |
| A. pontica (=
kessleri) |
60-73, mean 66.8 |
4.3-6.5, mean 5.5 |
| A. saposchnikowii |
20-48, mean 32.8 |
6.0-9.3, mean 7.3 |
The general Farsi name for these fishes is shag mahi or zalun (both
in Gilaki).
These herrings migrate from the north Caspian Sea to overwinter in the
central and southern parts, returning north in the spring.
Alosa braschnikowii
(Borodin, 1904)
Common names
shagmahi, shagmahi-ye Khazari.
[dolkii siyanayn, Agraxan siyanayi, Sara siyanayi, irikoz siyanak,
hasangulu siyanayi, agbas siyanak, all in Azerbaijan; Caspian marine
shad, Kurinskaya sel'd or Kura herring, poloschataya sel'd or striped
herring, Agrakhanskaya sel'd or Agrakhan herring, bol'sheglazaya sel'd
or bigeye herring, dolginskaya sel'd or dolginka herring, belogolovaya
sel'd or whitehead herring, Astrabadskaya sel'd or Astrabad herring,
sel'd-gonets or driver, zheltospinka or yellow-back, Gasankulinskaya
sel'd or Gasan-Kuli herring, kiselevichevskaya sel'd or Kiselevitch
herring, Krasnovodskaya sel'd or Krasnovodsk herring, vostochnaya
sel'd or eastern herring, obzhorka or glutton, Sarinskaya sel'd or
Sara herring, maiskaya sel'd or May herring, Brashnikovskaya sel'd or Brashhnikov's shad, all in Russian].
Systematics
Originally described as Clupea caspio-pontica var. Braschnikowii.
Reshetnikov et al. (1997) revert to the original double "i"
ending to the specific name. A lectotype from Fort Shevchenko (Aleksandrovsk) is in
the Zoological Institute, St. Petersburg (ZISP 13051) and
paralectotypes were designated by Svetovidov (1952)(ZISP 13051). Clupea
caspio-pontica is an unneeded new name according to Eschmeyer et al. (1996).
Alosa braschnikowii is regarded as a subspecies of Alosa
caspia by some authors. Clupeonella leucocephala Berg, 1913
from Sumgait and Gyurgenchai, Azerbaijan is a synonym (as Caspialosa
brashnikovi leucocephalia (sic) it is listed as a synonym
of C. b. grimmi in Mikhailovskaya (1941)), as is Caspialosa
caspia nigra Kisselevitsh, 1923 from the Caspian Sea opposite
Dzambai (the material also included specimens of Alosa
saposchnikowii) (Whitehead, 1985; Eschmeyer et al., 1996).
Alosa braschnikowii has 9 subspecies in the Caspian Sea
(including Alosa curensis (q.v.) the Kura or striped herring),
namely agrachanica (Mikhailovskaya, 1941) (author also spelt
Mikhaylovsky or Mikhailovsky; dated 1940 in Eschmeyer et al.
(1996) here and below but 1941 on the paper itself and in Svetovidov
(1952) and Berg (1948-1949); species also spelt agrakhanika in
Berg (1948-1949); Caspialosa brashnikovi morpha elata is
a synonym according to Mikhailovskaya (1941)), the Agrakhan herring; autumnalis
(Berg, 1915), the bigeye herring; braschnikowii (Borodin, 1904)
(also spelt brashnikovi in Svetovidov (1952) and Berg
(1948-1949)), the dolginka herring; grimmi (Borodin, 1904), the
whitehead or Astrabad herring, driver or yellow-back; kisselevitshi
(Bulgakov, 1926) (spelt kisselevitschi on the plate in Bulgakov
(1926), kisselevitschi in Mikhailovskaya (1941), kisselevitshi
in Svetovidov (1952) and Whitehead (1985) and kisselewitschi in
Berg (1948-1949)), the Gasan-Kuli or Kiselevitch herring; nirchi
(Morosov, 1928)(author also spelt Morosow in Mikhailovskaya (1941) and
Morozov in Eschmeyer et al. (1996)) (with Caspialosa
brashnikovi kenderlensis Budamshin, 1938 from Kendyrli Bay as a
synonym in Svetovidov (1952) and Berg (1948-1949)), the Krasnovodsk
herring; orientalis (Mikhailovskaya, 1941), the eastern herring
or glutton; and sarensis (Mikhailovskaya, 1941), the Sara or
May herring. Caspialosa brashnikovi derzhavini Tarasevich, 1946
described from the Caspian Sea near the Apsheron Peninsula, Azerbaijan
may be another subspecies. Caspialosa kiselevitschi morpha elata
Morozov, 1928 from the Caspian Sea, Krasnovodsk Bay, Turkmenistan is
an infrasubspecific taxon and its availability and validity as a taxon
have not been examined (Eschmeyer et al., 1996).
This high number of subspecies is an indication of the populational
variation of this shad and not all subspecies may be valid. A modern
revision is required to assess this problem. In light of this
uncertainty and the lack of adequate sample sizes to determine which
of the subspecies occurs in Iranian waters or which taxa are valid,
reference is made here mostly to the species level. Additionally, it
should be noted that hybrids between the various subspecies, and
between this species and other species, do occur to complicate matters even further.
The neotype of Caspialosa brashnikovi agrachanica
was designated by Svetovidov (1952) as the specimen described by Berg
as Caspialosa brashnikovi m. elata taken in front of the
Sulak River mouth, Agrakhan Bay and housed in the Zoological
Institute. St. Petersburg under ZISP 7334. However, this neotype was not validly
designated because qualifying conditions 75.3.1, 75.3.4 and 75.3.5 of the
International Code on Zoological Nomenclature were not met (N. G. Bogutskaya,
pers. comm., 23 January 2013). This also applies to the following 6 taxa.
The neotype of Caspialosa braschnikowi autumnalis was designated by Svetovidov (1952) as a
specimen 26.9 cm long from the eastern shore of the Caspian Sea at
Gasan-Kuli (just north of the Iranian border in Turkmenistan) caught
on 8 April 1948 and housed under ZISP 31749.
The neotype of Caspialosa kisselevitshi is also
from Gasan-Kuli caught on 30 June-1 July 1926 and was housed in the
Faculty of Zoology, Central-Asian State University (Sredne-Aziatskogo
Gosudarstvennogo Universiteta), Tashkent.
The neotype of Clupea caspio-pontica var. grimmi
was designated by Svetovidov (1952) as a specimen 34.0 cm long found
at Ashur-ade (= Ashuradeh) near Astrabad Bay (= Gorgan Bay or Khalij-e
Gorgan) on 23 April 1903 is under ZISP 13045.
The neotype of Caspialosa nirchi as designated by
Svetovidov (1952) is from the southern part of the Caspian Sea
opposite North Cheleken Spit and is under ZISP 31780.
The neotype of Caspialosa brashnikovi
orientalis as designated by Svetovidov (1952) is from the southern
part of the Caspian Sea opposite Kara-Ashly and is under ZISP 32187.
The neotype of Caspialosa brashnikovi sarensis from Sara Island is under ZISP 32184 as
established by Svetovidov (1952).
The lectotype of Clupea curensis from the Kura River estuary
is under ZISP 13984 with many paralectotypes as established by
Svetovidov (1952) (Eschmeyer et al., 1996).
Key characters
Characterised by a relatively elongate and rounded body likened to
a "herring" shape, not as deep as in some related species
which are likened to a "shad" shape. Total gill rakers 18-49
and short (about equal to gill filaments in length, sometimes
shorter). Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-5 unbranched and 12-15, mostly 14, branched rays,
anal fin with 2-4, usually 3, unbranched rays and 10-20, mostly 18,
branched rays. Scales in lateral series 51-54. Teeth are
well-developed on the jaws, tongue and roof of the mouth.
The accompanying table summarises characters of the subspecies and
is taken from Svetovidov (1952) and Mikhailovskaya (1941) but
identification to subspecies should be done with the keys from these works. Some of the
characters used in the keys are not in the table as they do show
individual variation and are difficult to summarise. An example is the
nature of the gill raker (thin, thick, blunt, pointed, bent, straight,
curved, branched, broken off, forked, swollen at the tip, etc.);
another is the degree of protrusion of the lower jaw.
The subspecies grimmi is quite specialised in association
with its benthic mode of life, feeding mostly on gobies (Gobiidae). It
has a unique character in the well-developed callus on the tip of the
lower jaw which adults acquire from rubbing the jaw on the sea bed
while feeding, gill rakers are low in number as fine food is not
taken, and the tips are broken off, broadened, and split owing to
abrasion, and the rakers on the lower arch are reduced in number so
the first raker is far from the tongue base. The subspecies nirchi
is similar. In contrast, the subspecies kisselevitshi has a
high gill raker count, rakers are pointed and not split at the tips,
and the first raker is close to the tongue base. This species lives in
surface waters feeding on Clupeonella, Atherina,
shrimps, gammarids, and gobies (Gobiidae).
|
Character /
Subspecies |
Gill rakers (mostly) |
Pectoral fins as % body length |
Vertebrae |
Head length as % of body length |
|
agrachanica |
20-46
(28-33) |
13.1-15.6 |
47-54 |
22.6-25.2 |
|
autumnalis |
21-37
(28-30) |
16.4-19.9 |
45-53 |
26.0-29.2 |
|
braschnikowii |
24-47
(30-33) |
14.3-16.7 |
48-55 |
23.5-26.6 |
|
curensis |
26-54 |
17.3-18.8 |
47-52 |
25.7-26.5 |
|
grimmi |
18-28
(20-22) |
12.9-15.2 |
45-52 |
22.9-26.4 |
|
kisselevitshi |
29-49
(36-40) |
13.9-16.8 |
43-53 |
24.2-26.9 |
|
nirchi |
20-31
(23-26) |
10.9-14.7 |
48-52 |
23.4-26.3 |
|
orientalis |
20-35
(27-32) |
13.5-18.0 |
45-53 |
25.0-27.8 |
|
sarensis |
20-33
(24-27) |
14.1-16.2 |
45-53 |
23.8-26.6 |
Sexual dimorphism
None reported.
Colour
The back and top of the head are dark with a green or blue tint and
may be grey-green. Some subspecies are paler in colour with a grey or
grass-green back and pale flanks, nirchi has a whitish
blue-green head, light grey back with a slight greenish tint, and
lower jaw and pectoral fins light, while grimmi is also quite
pale with a grey-blue back and top of the head and whitish anterior
head and pectoral fins. There is a dark spot behind the operculum but
no series of spots along the flank in most subspecies, except in rare
cases when there may be up to 7, occasionally 12-13. The subspecies grimmi
regularly has a row of diffused, grey spots almost merging into a
stripe, and nirchi occasionally. Pectoral fins are dark on some
subspecies (braschnikowii, sarensis, kisselevitshi),
pale or whitish on the others, although there is confusion in the
literature over this, perhaps indicative of individual variation (cf. sarensis
in Mikhailovskaya (1941)). The back and upper part of the head may become a deep
black at spawning. The flanks and belly are silvery.
Size
Attains 50 cm standard length but average lengths are about 27-34 cm.
Distribution
All the Caspian subspecies are found widely distributed in the sea
but chiefly in the south in winter, moving north to spawn in spring.
The subspecies sarensis is reported from the Lenkoran coast and
from southwest of Gasan-Kuli (in Turkmenistan just north of the
Iranian border), the subspecies orientalis from Gorgan or
Astrabad Bay, autumnalis from coastal waters at Gasan-Kuli, kisselevitshi
from Astara and Gasan-Kuli, and grimmi from Astara and Gorgan Bay.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
In winter this species moves into deeper water towards the Iranian
coast. In March it approaches coastal waters (Vetchanin, 1984)
including brackish waters but does not enter fresh water. It never enters rivers
in the south of the Caspian Sea (Jolodar and Abdoli, 2004). Salinities
up to 47.6‰ are survived by this species. Spawning and feeding
grounds are in the north Caspian for some populations but others live
permanently in the south Caspian Sea and are of smaller size. The
subspecies kisselevitshi, for example, lives off Gasan-Kuli in
winter at depths below 25 m, not migrating or feeding. In March they
move north to feed and then return south to spawn but lives almost
entirely as a pelagic species in the southern Caspian Sea. Knipovich
(1921) reports this species from depths of 80-98 m in Iranian waters. The
density of this species increased from east to west in a 1999-2001 study in
Iranian waters (Afraei, 2006). Abdoli and Naderi (2009) list it as from the
southwest, southeast and south-central Caspian Sea in Iranian waters.
Age and growth
Maturity is attained at age 2-5 and life span is up to 10 years,
although this varies with the subspecies. Most south Caspian forms
apparently mature at age 2 according to Svetovidov (1952). Growth
rates also vary between subspecies, orientalis being one of the
slowest growing herrings in the Caspian Sea and reaching 10 years of
age. The catch near Astara of sarensis, for example, is mainly
4-5 year olds but this too varies with the subspecies and also with
the year-class strength. Vetchanin (1992) reported on grimmi
catches from the southeastern Caspian where the average length was
27.8-28.6 cm and the average weight 294-313 g. There is a tendency for
length and weight to fall in catches as the summer progressed, from
April to July. Length and weight are less in southern, compared to
northern, waters.
Afraei (2000) found this species to be the largest Alosa in Iranian
waters on average at 395 mm and 760.3 g. Males predominate at 55.8% in Mazandaran
and 69.4% in Golestan catches. Six age classes were present (1+ to
6+) with the 2+ class being the most common at 28.9% and 6+ the rarest at 8.9%.
Food
Diet in the southeastern Caspian Sea in winter comprises 85% Clupeonella
engrauliformis with some gobies (Neogobius) and shrimps (Vetchanin,
1984). From March to November the diet is dominated by Clupeonella
caspia, Atherina boyeri (= caspia) and shrimps. Juvenile Liza
saliens, Syngnathus caspius, molluscs, crabs and higher
aquatic plants are also recorded along with foreign objects such as
rice husks, pieces of wood, foil, polyethylene, etc. This species is a
cannibal. The more southerly populations examined favour Atherina
boyeri (= caspia) and Neogobius species and some of these populations
favour benthic invertebrates. The subspecies grimmi is the most
benthic one and takes primarily gobies with some molluscs as well as Clupeonella.
Feeding intensity rises sharply after spawning. While some herrings,
like Alosa pontica (= kessleri), feed poorly on their migration, this
species feeds intensively on its spring migration.
The feeding regime altered after the invasion of the ctenophore,
Mnemiopsis leidyi. A shift was observed from 85% Clupeonella
engrauliformis to 65% Atherina boyeri (= caspia). Other fishes were also eaten
including Clupeonella grimmi, C. caspia, Cyprinus carpio,
Liza saliens, as well as Palaemon spp. (Iranian Fisheries
Research Organization Newsletter, 49:2, 2006).
Reproduction
Vetchanin (1984) reports spawning of this species in the
southeastern Caspian Sea north of Iranian waters to begin in early
May, continuing to July as it is intermittent. The subspecies sarensis
spawns along the Lenkoran coast from mid-April to the end of June. The
subspecies orientalis spawns in Gorgan Bay from the end of
March to the beginning of April, spawning schools forming at 17-18°C
or higher. The subspecies autumnalis spawns at the same time
off Gasan-Kuli near the Iranian border with Turkmenistan. The
subspecies grimmi spawns in May-June in Gorgan Bay. The
subspecies kisselevitshi has the latest spawning date, June to
July and even in August off Gasan-Kuli when temperatures exceed 25°C.
Spawning takes place in shallow water (1.8-5.8 m) in the sea over sand
or silt bottoms at 15-18°C (some subspecies and populations at 20-22°C,
others beginning as low as 11°C), and a salinity of 8-13‰. Fecundity is up to 178,400 eggs, average
66,000 per fish. There is no feeding while spawning. Early maturers,
like the south Caspian populations, can reproduce up to 7 times in their life.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
The catch for all species of "Caspialosa" in Iran
varied between 5337 kg and 419,518 kg for the years 1956/1957 to
1961/1962 (Vladykov, 1964). In the Anzali region the catch for the
years 1933/1934 to 1961/1962 varied from 1553 kg to 539,710 kg (Vladykov, 1964).
The catch has been as high as 126,900 centners or 12,690 t in the
sea as a whole for the type subspecies alone (1 centner = 100 kg (Svetovidov,
1952)), taken chiefly in spring. Other subspecies were not fished for
as extensively although kisselevitshi was the most numerous of
the south Caspian forms of Alosa braschnikowii, forming 70% of
the drift net catch.
Conservation
Reputedly depleted in Iranian waters. Kiabi et al. (1999)
consider this species to be data deficient in the south Caspian Sea
basin according to IUCN criteria. Criteria include medium numbers,
medium range (25-75% of water bodies), absent in other water bodies in
Iran, and present outside the Caspian Sea basin. Extinct in Turkey (Fricke et
al., 2007).
Further work
The biology of this species in Iranian waters and the stocks or taxa found
there need to be elucidated.
Sources
Iranian material: CMNFI 1970-0581, 5, 226.0-245.0 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1979-0431, 1, 297.2 mm standard length, Mazandaran, bazaar at Now Shahr (no other locality data);
CMNFI 1980-0126, 1, 245.8 mm standard length Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0150, 1, 222.4 mm standard length, Gilan, Safid River estuary (37º24'N, 49º58'E).
Comparative material: BM(NH) 1938.8.2:1, 1, 245.9 mm standard length,
Kazakhstan, Caspian Sea, Kaidak Bay (no other locality data); BM(NH) 1938.8.2:2, 1, ca. 337.5 mm standard length,
Kazakhstan, Caspian Sea, Kaidak Bay (no other locality data); BM(NH)
1939.2.21:17-18, 2, 285.0-305.2 mm standard length, Caspian Sea (no other
locality data); BM(NH) 1939.2.21:19-20, 2, 222.9-273.4 mm standard length,
Caspian Sea (no other locality data).
Alosa caspia
(Eichwald, 1838)
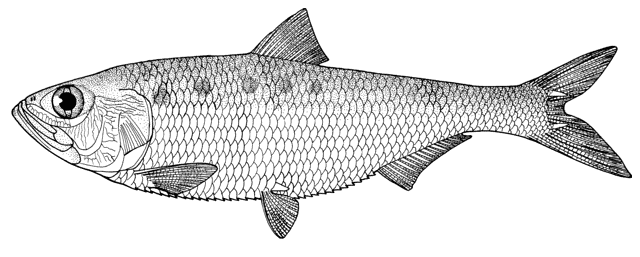
Common names
shagmahi-ye shekambozorg (= big belly herring fish), shagmahi-ye chekameh dar, shagmahi-ye darya-ye
khazar (= Caspian Sea herring fish), شاه ماهي (= shah mahi, meaning king fish),
zalun (in Gilaki), puzanok.
[xazar sisgarini, sara sisgarini in Azerbaijan; Kaspiiskii puzanok
or Caspian shad, severokaspiiskii puzanok or North Caspian shad,
srednekaspiiskii puzanok or Central Caspian shad, il'mennyi puzanok or
il'men shad, Enzeliiskii puzanok or Enzeli (= Anzali) shad, Sarinskii
puzanok or Sara shad, Bakinskii puzanok or Baku shad, Astrabadskii
puzanok or Astrabad shad, all in Russian].
Systematics
Clupea caspia was originally described in Latin from "Hab.
in Caspio mari, meridiem versus" (Caspian Sea, towards the south).
A. caspia has 3 subspecies in the Caspian Sea basin, namely caspia
(Eichwald, 1838) (= North Caspian, Central Caspian, Caspian or il'men
shad); knipowitschi (Iljin, 1927) with natio knipowitschi
(Iljin, 1927) (= Enzeli or Anzali shad) and natio saraica (Svetovidov,
1943) (= Sara or Baku shad); and persica (Iljin, 1927) (=
Astrabad shad). The differences between natio knipowitschi and
natio saraica are small (e.g. gill rakers 122-166 versus
140-150, both averaging 145; vertebrae 43-49 versus 45-51, both with
mostly 47 or 48; growth differences are known, the former grows faster
in the first 2 years of life but the latter reaches a greater size)
and they probably have no taxonomic significance being simply separate
breeding populations. The differences between Alosa caspia caspia
natio caspia (the North or Central Caspian shad) and natio aestuarina
Berg, 1932 (the il'men shad) were found to be based on geography and
growth rate and these names have no taxonomic standing (Svetovidov,
1952). These natio are infrasubspecific ranks and have no validity as names.
Alosa rossica Kessler, 1870 described from the Volga River
is a nomen nudum and is this species. Other taxa now considered
as synonyms of Alosa caspia are Caspialosa caspia salina
Svetovidov, 1936 from Mertvyi Kultuk and Kaidak bays in the northeast
Caspian Sea and Caspialosa caspia kaidakensis Kazancheev, 1936
(spelt kajdakensis in Svetovidov (1952)) from Kaidak, the
latter being in any case a synonym of the former subspecies. Clupeonella
caspia m. elongata Berg, 1913 is also a synonym. Alosa
caspica Yakovlev, 1871 is presumably a misspelling.
Knipovich (1921) records a species, Caspialosa enzeliensis
Iljin, from the southern Caspian Sea which he places as a subspecies
of caspia. I have been unable to locate the original
description of this taxon, which presumably is found in the Anzali
Mordab of Iran. It is probably an unused manuscript name for what
Iljin later described as knipowitschi.
As of 15 July 2007, this scientific name is a Googleblat for this page.
The lectotype of Caspialosa knipowitschi is a specimen 21.2
cm long from Anzali in Iran caught on 15 April 1915 and housed in the
Zoological Institute, St. Petersburg (ZISP 31892). The lectotype of Caspialosa
caspia var. persica is a specimen 147.5 mm long from the
Caspian Sea Bay of Asterabad (= Gorgan Bay or Khalij-e Gorgan) north
of Ashur-ade (= Ashuradeh) at 36°53'N, 53°55'E
caught on 25 April 1904 on the Caspian Expedition of 1904 and housed
in the Zoological Institute, St. Petersburg (ZISP 16413). The
lectotype of Caspialosa caspia knipowitschi n. saraica
is from near Sara Island and is under ZISP 32183. The lectotype of Caspialosa
caspia salina is from Mertvyi Kultuk Bay, 10 km west of Cape
Kizil-kair and is under ZISP 25813. These taxa were designated by
Svetovidov (1952) as none were before or material was not preserved.
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. Total gill
rakers 50-180, variously reported as thin or thick, long (obviously
longer than the gill filaments), and forming a convex outline on the
lower arch. Teeth are poorly developed in both jaws.
Morphology
Dorsal fin with 3-4 unbranched and 12-15 branched rays, anal fin
with 3-4, usually 3, unbranched and 15-20 branched rays. Scales in
lateral series 49-54.
The characters distinguishing subspecies all overlap widely and are
given below after Svetovidov (1952) and Hoestlandt (1991):-
|
Characters /
Subspecies |
Head length as % of body length |
Pectoral length as % of body length |
Vertebrae |
Gill rakers |
|
caspia |
25.5-28.1 |
15.5-18.1 |
45-52
(49-51) |
68-150
(100-140) |
|
knipowitschi |
18.3-24.1* |
16.0-19.1 |
43-51
(47-48) |
120-180
(130-160) |
|
persica |
25.6-27.1 |
16.5-17.7 |
45-51
(47-49) |
50-120
(60-90) |
* The numbers cited in Svetovidov (1952: 256 in the
English version) and Hoestlandt (1991: 128) in the keys to subspecies
do not agree with the numbers on p. 148 and p. 265 respectively in the
species descriptions. The text numbers are used here.
Sexual dimorphism
Females are longer and weigh more than males of the same age.
Colour
The back is blue-green to dark and the flanks
silvery. There is a black spot on the flank behind the upper operculum
margin and sometimes up to 7 spots extending along the upper flank to
a level of the rear of the dorsal fin.
Size
Reaches 28 cm standard length for caspia, to
29.6 cm for knipowitschi, and to 33.8 cm for persica.
Distribution
Found in the Caspian and Black seas. The subspecies caspia
is found mostly in the western half of the Caspian Sea basin but is
the most widely distributed subspecies, found throughout almost the
whole sea. The subspecies knipowitschi is found in the south
near Anzali, Astara and the Baku Archipelago, near the northern shore
of the Apsheron Peninsula in autumn with a few reaching the Gorgan Mordab
in fall and winter; natio saraica is found north of Astara and
spawns near Sara Island, natio knipowitschi spawns in the
Anzali Mordab. The subspecies persica is found in the
southeast, near Gorgan or Astrabad Bay. Holčík and Oláh (1992) report persica from the western basin of the
Anzali Mordab (= Talab) and this species is reported from the Safid River and Anzali Talab as
subspecies persica and from the Anzali Talab as knipowitschi
(Abbasi et al., 1999).
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea, the Anzali Talab and Gorgan Bay in Iranian waters for
both knipowitschi and persica.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
The type subspecies prefers open waters. Caspian shad winter at depths of 30-40 m or more and prefer temperatures not
less than 8-11°C. They rise to surface waters in spring, moving north along the western
shore of the Caspian Sea in waters of about 9-11°C according to Kushnarenko (1986) while Heckman in Hoestlandt (1991) states that
this shad begins to migrate at the end of March at 5-6°C water temperature with a peak at 9-14°C
in mid to late-April, ending in early May. Males migrate in large numbers at the beginning and end of the migration, females in the
middle (Pushbarnek, 1987) while Heckman in Hoestlandt (1991) states that two waves of migration occur, one usually in late April at
7.6-10.2°C comprised of over 80% males and the second in the first half of May at 10.8-14.0°C
comprised of over 70% females. The young, which hatch in the spring, leave the summer feeding grounds before the adults and migrate south
before October-November. Adults follow as temperatures fall. Some populations do not migrate north and spend their whole life in the
southern Caspian Sea. This subspecies will enter fresh waters to spawn in addition to spawning in the open Caspian Sea.
The subspecies knipowitschia prefers water warmer than that of all other Caspian Sea clupeids except for Alosa
caspia persica. Its sea movements are not well known but spawning fish favour waters with freshwater input and some fish enter rivers so
it is classified as semi-anadromous. This subspecies was common in the Anzali Mordab but is now replaced by persica (Holčík and Oláh, 1992).
It is also reported westwards to Astara and eastwards to Gorgan Bay. The winter habitat of persica is unknown. It
is semi-anadromous and remains in the southern Caspian Sea near the shore. From spring to fall this subspecies moves northward along the
eastern Caspian shore towards Krasnovodsk Bay and westwards to the Anzali Mordab.
Age and growth
Pushbarnek (1987) found shad of the type subspecies
up to 7 years of age on the western coast of the middle Caspian Sea.
In the spawning population, the predominant sizes and weighs for males
were 16-21 cm and 60-130 g and for females 18-23 cm and 70-140 g.
Males and females usually mature at 2-3 years although most spawn for
the first time at 3 years. Females grow faster than males. Shad may
spawn up to four times as the period of sexual maturation may continue
for 2-5 years. The age composition of the spawning population is
dependent on year-class strength. First spawners constitute 75.9% of
3-year-olds, 41.7% of 4-year-olds and 23.5% of 5-year-olds. The
Caspian shad is a slow-growing species compared to A. braschnikowii
and A. saposchnikowii, its mean length being 21.2 cm compared
to 32.2 cm and 25.6 cm for the two other species respectively (Shubina, 1981).
Dmitriev (1947) briefly examined the Anzali, Iran
population and found 6 age groups but life span is noted by Heckman in
Hoestlandt (1991) to be up to 9 years. Maturity is attained as early
as 2 years although most fish appear to mature later as most spawners
are 4-5 years old.
The subspecies persica is the slowest growing
of the shad species in the Caspian Sea, sexually mature fish being 13-21 cm
long. Some fish become mature at 2 years of age. Life span is up to 8 years.
The populations of both knipowitschi and persica
are small compared to caspia.
Abbasi and Sabkara (2004c) studied 180 fish from the southeast Caspian Sea coast
of Iran and found fork length to be 103-232 mm, mean 158.8 mm, weight 16-130 g,
mean 52.2 g and age 2-5 years, mean 2.64 years. Afraei (2000) found this species
to be the smallest Alosa in Iranian waters on average at 110 mm and 109 g.
Patimar et al. (2011) found Iranian fish to reach age 5+ years, with
positive allometric growth in the southeast Caspian, and negative allometric
growth in the central and southwest Caspian. Females had a b-value larger
than males. von Bertlanffy growth functions were variable, different between
males and females of each area and between sexes from different areas. The
largest L∞ was in the southeast for males and in the central area for
females. The highest K value was for males and the lowest for females in the
southeast area. t0 was negative in all areas with lowest value for
females (-0.531) in the southeast and the highest for males (-0.145) in the
central area.
Food
The most intensive feeding period occurs after
reproduction, beginning in June and the highest condition factor is
found at the end of this summer feeding period. Little food is eaten
in winter. Temperature (affecting metabolic rate) and zooplankton
biomass (decreases engender competition with Clupeonella
engrauliformis and other planktivores) are important factors
governing catches of this species (Shubina, 1981). Food is chiefly
copepods, more than 70%, with mysids at 20%, but some phytoplankton
and small fishes are taken. Food in rivers after spawning is mostly
cladocerans and other crustaceans. The above refers to the type
subspecies; food of the other two subspecies is assumed to be similar. The
southeast Caspian Sea fish studied by Abbasi and Sabkara (2004c) fed on
phytoplankton (Rhizosolenia and Sprirogyra) at 4.5%, zooplankton
(Foraminifera, Copepoda, Cirripedia, Bivalvia larvae) at 95.0%, and bony fish
larvae and eggs at 0.5%. The presence of the ctenophore, Mnemiopsis leidyi,
a food competitor reduced the index of fullness and fish growth was reduced.
Abdollapour Bereya et al. (2007) studied diet in fish from beach seines
and gill nets in Gilan. 98.0% of the stomach contents were zooplankton (ostracods,
rhizopods, cladocerans, rotatarians, copepods, cirripedes, mysids, bivalve
larvae and bony fish larvae and eggs), 1.8% was phytoplankton (notably
Rhizosolenia and Spirogyra), and 0.2% was benhthic items (foraminiferans,
sponges, cumaceans, amphipods, insect lavae and palaemonids). Acartia spp.
(copepods) at 83.1% and Balanus (cypris larvae of the cirripede) at 12.9%
were the most abundant. The zooplankton have declined drastically from predation
by Mnemiopsis leidyi, the invasive ctenophore, and the fish have shown a
great reduction in the index of fullness and in growth recently.
Reproduction
Most spawning of the type subspecies occurs in the
north Caspian Sea near the outflow of the major rivers, particularly
the Volga, and the fish overwinter in the south Caspian, migrating
between the two areas (Shubina, 1981). This subspecies spawns
successively, 3 times within a week. Some fish enter fresh water to
spawn. Spawning takes place at the favoured water temperature of
13.8-24.1°C, with mass spawning at 18-22°C,
beginning as early as late April or as late as mid-May and continuing
to mid- or late June. Most eggs are released in the upper 3 m of the
water column. Fecundity reaches 41,000 eggs. The eggs are 1.11-1.38 mm
when ripe but unfertilised and 1.92-2.91 mm in diameter when
fertilised and are semi-pelagic to demersal.
The subspecies knipowitschi spawns in the
Anzali Mordab (and probably the "Chemkhala" River to the
east of the Safid River) in May and June after a spring migration from
the sea, leaving in the fall. Spawning of the subspecies persica takes
place in Gorgan Bay and Holčík and Oláh (1992) suspect from catches of mature and spent fish that it
also occurs in the Anzali Mordab.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator
on this species (Krylov, 1984) and it forms a substantial part of the
diet of Silurus glanis in the Anzali Mordab (Holčík and Oláh, 1992).
Ashoori et al. (2012) found that grey herons (Ardea cinerea) in
the Siahkeshim Protected Area of the Anzali Wetland ate this species.
Naem et al. (2002) found the monogenean trematode Mazocraes alosae
on the gills of this species in the western branch of the Safid River.
Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Youssefi et al. (2011) report the digenean Pronoprymna ventricosa
from fish in the Babol River. Barzegar et al. (2012) found Mazocraes
alosae, Diplostomum spathaceum (monogeneans), Pronoprymna
ventricosa (digenean), and Hysterothylacium sp. (anisakid).
Economic importance
The type subspecies was the most important
subspecies in the herring family in the Caspian Sea. It is caught off
the coasts of Dagestan and Azerbaijan for research purposes and
comprises 85% of the clupeid catch (Pushbarnek, 1987), 80-90% of the
Caspian commercial catch (Kushnarenko, 1986). During the 1970s it was
only 2% of the total Caspian fishery production. These herrings
dominated the commercial catch in the Caspian Sea until the 1960s when
commercial fishing was banned except on the western coast of the
central Caspian. Many young of other commercial species were being
killed in the herring fishery, entangled in the gill nets used. Soviet
catches have weighed as much as 75,000 t. This fish is fattier than
other Caspian Clupeidae, except for Alosa kessleri, up
to 18.1% of the body weight. The fat content decreases on the spring migration.
The catch of the subspecies knipowitschi is
of minor economic importance and had been little exploited when
Svetovidov (1952) summarised biology, as the age of captured fish
indicated. About 420 tons (sic, possibly tonnes) were caught in
the Anzali Mordab in 1933 and 1934, but this may be an error in the
report by Vladykov (1964) according to Holčík and Oláh (1992) although Berg (1948-1949) reports 4200 centners for
the same period. The fishing season in the mordab began in mid-April
and ended in mid-June when spent fish appeared. There appears to be no
fishery data on the subspecies persica in the sea. Holčík and Oláh (1992)
report catches of persica, which replaced knipowitschi,
in the Anzali Mordab from the end of April to the beginning of June
but in 1990 this comprised only 5 kg. It is regarded as of inferior quality in Iran.
The Caspian shad is the dominant fish catch in the
Iranian Caspian, comprising 51,000 t in 1994 rising from nothing
a decade earlier (Food and Agriculture Organization, Fisheries Department, 1996).
Robins et al. (1991) list this species as
important to North Americans. Importance is based on its use as food.
Conservation
The stocks of this species in the Anzali Mordab are
likely to increase as the lagoon becomes more saline (Holčík and Oláh, 1992).
Kiabi et al. (1999) consider this species to
be of least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include abundant in numbers, widespread range (75%
of water bodies), absent in other water bodies in Iran, and absent
outside the Caspian Sea basin. Extinct in Turkey (Fricke et al., 2007).
Further work
The biology of this species in Iranian waters and the stocks or taxa found there need to be elucidated.
Sources
See under family heading.
Iranian material: CMNFI 1970-0524, 11, 58.7-88.9 mm standard length, Gilan, Caspian Sea at Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0532, 1, 113.0 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 1, 85.9 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0586, 1, 77.5 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak (36º50'N, 53º56'E);
CMNFI 1970-0587, 2, 107.4-108.6 mm standard length, Mazandaran, Babol Sar (36º43'N, 52º39'E);
CMNFI 1971-0343, 1, 95.5 mm standard length, Gilan, Langarud at Chamkhaleh (37º13'N, 50º16'E);
CMNFI 1979-0430, 1, 118.0 mm standard length, Mazandaran, river east of Now Shahr (36º39'N, 51º31'E);
CMNFI 1979-0431, 7, 120.8-155.1 mm standard length, Mazandaran, Now Shahr bazaar (no other locality data);
CMNFI 1979-0686, 2, 119.7-126.9 mm standard length, Gilan, Safid River (37º24'N, 49º58'E);
CMNFI 1980-0146, 2, 106.9-171.8 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak (36º50'N, 53º56'E).
Comparative material: BM(NH) 1938.8.2:3, 1, 203.8 mm standard length, Caspian
Sea (no other locality data); BM(NH) 1939.2.21:22-23, 2, 175.6-179.2 mm standard
length, Caspian Sea (no other locality data); BM(NH) 1954.6.24:5-7, 3, 164.1-189.1 mm standard
length, Caspian Sea (no other locality data).
Alosa curensis
(Suvorov, 1907)
This species is poorly known and not recorded from Iran but from
Kyzylagach Bay of Azerbaijan. It may, in any case, be a subspecies or
synonym of Alosa braschnikowii (see Svetovidov (1952) and the Alosa
braschnikowii account herein).
Alosa kessleri
(Grimm, 1887)
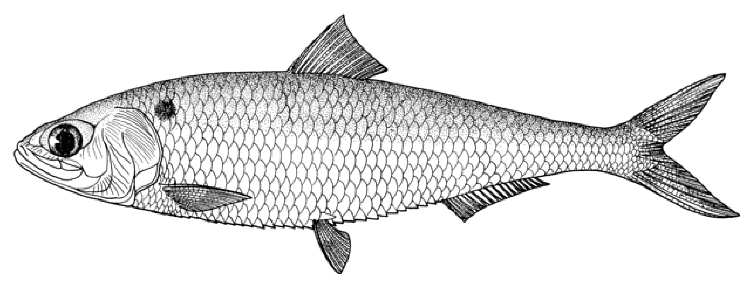
Common names
shagmahi-ye poshtsiah, shagmahi darya-ye siah, shagmahi-ye moohajer or
shagmahi-e-mohajer, zalun (in Gilaki), puzanok.
[Volga siyanayi, garabel siyanak in Azerbaijanian; arkasy gara takgas in
Turkmenian; blackback, Caspian
anadromous shad; chernospinka or black-spined herring, chernonosik or
blacknose, beshenka, zalom, poluzalom, zheleznitsa, veselka,
Volzhskaya mnogotychinkovaya sel'd or Volga many-rakered herring,
Volzhskaya sel'd or Volga herring, Astrakhanskaya sel'd or Astrakhan
herring, all in Russian; Pontic shad, Black Sea herring].
Systematics
Clupea kessleri was originally described from the Volga River delta,
Astrakhan. Clupea pontica was originally described in Latin from "Hab.
in Ponte Euxino prope Odessam" (= Black Sea near Odessa).
Alosa kessleri was formerly considered as a subspecies of A.
pontica. Alosa pontica then had two subspecies in the Caspian Sea, namely kessleri
(Grimm, 1887) (chernospinka or black-spined herring, chernonosik or
blacknose, beshenka, zalom, poluzalom, zheleznitsa, veselka, blackback),
and volgensis (Berg, 1913) (Volzhskaya mnogotychinkovaya sel'd
or Volga many-rakered herring, Volzhskaya sel'd or Volga herring,
Astrakhanskaya sel'd or Astrakhan herring, zheleznitsa, beshenka,
veselka). Kottelat and Freyhof (2007), Abdoli and Naderi (2009) and Naseka
and Bogutskaya (2009) consider Alosa kessleri and A. volgensis to
be valid species.
A lectotype of kessleri, 40.1 cm long, was designated from
the Volga Delta by L. S. Berg under ZISP 15925 (in the Zoological
Institute, St. Petersburg). A lectotype of volgensis, 34.8 cm
long, is under ZISP 15926 and is from the Volga River at Chernyi Yar (Svetovidov, 1952).
A paralectotype of kessleri is under ZIN 15922.
Caspialosa volgensis bergi Tanasiichuk, 1938 described from
the Volga Delta is a synonym of Alosa kessleri (Heckman
in Hoestlandt, 1991). Eschmeyer et al. (1996) give author and
date for Alosa volgensis bergi as Tanassiychuk, 1940, the
variation probably being due to transliteration of a Russian name and
to year of actual publication rather than year on the journal.
Caspialosa kessleri infraspecies volgensis imitans
Berg, 1948 from the Caspian Sea (see Berg (1948-1949) for further
details) is not available because of its infrasubspecific rank (Eschmeyer
et al., 1996).
Clupea caspio-pontica Borodin, 1904 is an unneeded new name
for these fishes from the Black and Caspian seas (Eschmeyer et al., 1996).
Key characters
Characterised by a relatively elongate and rounded body likened to
a "herring" shape, not as deep and compressed as in some
related species which are likened to a "shad" shape. Total
gill rakers 57-158 in the Caspian Sea, 57-95 in kessleri,
87-158 in volgensis. Rakers are usually longer than the gill
filaments in volgensis, shorter in adult kessleri. Teeth
are well developed in both jaws in kessleri and can be felt
with a finger, poorly developed in volgensis such that they
sometimes cannot be felt.
Morphology
Dorsal fin with 3-5 unbranched and 12-16 branched rays, anal fin
with 2-4, usually 3, unbranched and 15-21 branched rays. Vertebrae
47-50 in kessleri (also a report of 50-54, both in Svetovidov
(1952)), 48-54 in volgensis. Pyloric caeca 21-62. Scales in
lateral series 53-56. Gill rakers in adults are thick and often broken
off at the tip or near the base in kessleri, unbroken in volgensis.
The tips of the gill rakers may be swollen and they are arranged in a
straight line. Young fish have long and thin gill rakers with strong
lateral spines. Spines are lost with age. Chromosome number is 2n=48 (Klinkhardt
et al., 1995).
Sexual dimorphism
None reported.
Colour
The overall coloration is dark with a black back which has a violet
tinge in spring in kessleri, light olive green in volgensis.
There is dark, sometimes vague, spot on the flank behind the operculum
and sometimes a series of spots in kessleri, but these are
absent in volgensis. The pectoral fin is black on top. Spawning kessleri
become grey or grey-green on the back and flanks with bronze spots on
the operculum and flanks. A greenish-yellow circle forms around the
eye after spawning.
Size
Reaches 52 cm total length and 2.0 kg for kessleri, 40 cm for volgensis.
Distribution
Found in the Black and Caspian seas and throughout the latter, entering northern rivers to spawn.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
Both subspecies are found in the open sea but kessleri
ascends rivers much higher than volgensis which spawns in the
delta region. Both subspecies overwinter in the southern Caspian Sea
off the Iranian coast and then migrate north to enter the Volga and
other northern rivers to spawn. The subspecies volgensis is
absent from the southern Caspian in summer. The subspecies kessleri
shows a greater affinity than volgensis for cold water.
The subspecies kessleri begins to migrate northward in March
and April mostly along the western shore of the Caspian Sea, beginning
to arrive in northern waters when temperatures are still below 5°C,
most arriving when temperatures are 6-8°C compared to 10-13°C
for volgensis. A mass migration into the lower Volga takes
place in late April or early May for both subspecies when water
temperature reaches 9°C and the peak run begins at 12-15°C,
ending at 22°C. The run of volgensis is about 10 days later than that of kessleri
and spawning takes place earlier as they do not travel as far upriver.
Speed is up to 70 km/day for kessleri and depends on
temperature. This fish used to run 2000 km up the Volga River. Sexually immature fish remain in the south and do not
migrate. Knipovich (1921) reports kessleri as deep as 235-300 m in Iranian waters.
Temperatures up to 25ºC are tolerated.
Age and growth
Males are sexually mature at 3 years and females at 4 years, other
reports give 4-5 years for both sexes in kessleri. Many fish
die after spawning but some survive to spawn two or three times. Four
and five-year- olds dominate on kessleri spawning runs with
some older fish also present. Females predominate in older fish making
the spawning run. Life span is between 7 and 8 years.
Growth of the volgensis subspecies is slower than in kessleri,
which apparently grows faster than any other Caspian clupeid. Life
span in volgensis is 7-8 years with females living longer than
males. Most spawners are 3-4 years old although in some years 5 year
old fish are abundant. Males may mature at 2 years, females later.
Most fish spawn again the next year after their first time but some
may miss a year. An individual may spawn up to four times during its life.
Yılmaz and Polat (2002) compared scales,
vertebrae, otoliths, opercles and subopercles as ageing structures and
determined vertebrae to be the most accurate and reliable for a Turkish Black
Sea population at Samsun. Six age classes were found.
Food
Cladocerans are the main food item of young kessleri which
have a feeding peak at 1800-2200 hours and another at about 0800
hours. Adults in the sea take fishes such as Clupeonella and Atherina
with some crustaceans and insect larvae. Clupeonella caspia
makes up 92% of the diet of kessleri in the northern Caspian in
May, with Sander lucioperca at 6.6% and gammarids at 1.0%.
There is said to be little feeding on the spawning run although some
fish sampled contained cladocerans, copepods, insects, bryozoans and fish fry.
The food of volgensis is similar to the other subspecies,
taking copepods when young and larger items with growth. The main
items are copepods, mysids, cumaceans, amphipods and small fishes.
This subspecies feeds on the spawning migration.
Reproduction
Spawning in kessleri occurs in rivers from mid-May to
mid-August, either the delta or lower reaches when entering in a ripe
condition, or as much as 500 km upriver when entering in an unripe
condition. Larger fish have spawning grounds further upriver than
smaller fish and predominate earlier in the run. The spawning grounds
in the Volga River cover a considerable stretch. Spawning usually
occurs at 18-20°C
between 0300 and 0600 hours or from 1600 hours to sunset. Spawning
occurs in the main channel, over shallow sand banks, or in backwaters.
Batches of eggs are laid at intervals of several days. Eggs are
pelagic as in other Caspian Alosa and develop as they drift
downriver near the bottom. At 22.7°C
incubation takes about 40 hours. The young fish descend in late summer
and early fall. Fecundity in kessleri reaches 344,000 eggs and
egg diameter 1.51 mm. Shed eggs are up to 4.1m in diameter. Some fish may return to spawn in total three times.
Spawning of the first batch of eggs in volgensis may occur
in the sea with the subsequent 2 batches at 7-10 day intervals in the
delta and river. This takes place from mid-May to the beginning of
August. Up to 281,000 eggs are shed. Peak spawning occurs at 15-19°C
and ends at 25-27°C. Most spawning takes place in the evening between the 1600 and 2200
hours. The young appear in the pre-estuarine area of the Volga River
in July and towards October begin to migrate south.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984), larval shad are fed on by other fishes and by various
invertebrates, and adults by various fishes and birds.
Economic importance
The subspecies kessleri and volgensis were caught on
the spawning run with as much as 5750 t being taken annually pre-World
War II. It is the biggest shad in the Caspian Sea. The subspecies kessleri was the most important and
valuable herring in the Caspian Sea. Early spring catches were mostly kessleri
but as the run of volgensis built up it formed an increasingly
significant part of the catch, forming as much as 92% of the total.
The catch of volgensis has declined from this period until the
1970s when the fish taken were mostly kessleri. The catch of Alosa
pontica (= kessleri) on the North Caspian fishing grounds in 1965-1972 has
declined to 2-4% of the 1938-1943 catch. The subspecies volgensis
was one of the most important Caspian herrings, 23-29% of the total
catch from 1936-1939, as high as 69,100 t in 1939.
The subspecies kessleri is said to be the tastiest Caspian
clupeid because of its high fat content, averaging 18.9% of weight
along the coast of Azerbaijan, while in volgensis it was 9.6%.
Post-spawners of kessleri may have a fat content as low as 0.5%.
Catches are processed as canned, salted and pickled fish. Beach seines are used
to catch this fish. Akhondzadeh Basteh et al. (2006) found the bacterial
pathogen Vibrio haemolyticus in fresh and smoked Alosa kessleri.
Tavakoli et al. (2012) found a frequent contamination rate with
Staphylococcus aureus and Vibrio parahaemolyticus, human pathogens,
in fresh and smoked shad and they may cause health problems.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food.
Conservation
Stocks in Iranian waters are said to be depleted. The subspecies volgensis
was in Category I on the "Red List" of the Russian Republic
(Pavlov et al., 1985). Kiabi et al. (1999) consider this
species (as A. kessleri) to be data deficient in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, numbers unknown, range unknown absent in other
water bodies in Iran, absent outside the Caspian Sea basin.
Further work
Stocks in Iranian waters need to be assessed and protected if required.
Sources
See under family account.
Iranian material:
None available.
Comparative material: BM(NH) 1879.11.14:22-23, 2, 255.9-259.1 mm standard
length, Caspian Sea (no other locality data); BM(NH) 1939.2.21:21, 1, 388.6 mm
standard length, Caspian Sea (no other locality data).
Alosa saposchnikowii
(Grimm, 1887)

Common names
shagmahi-ye cheshmdorosht, shagmahi, kilka (incorrectly), herring.
[irikoz sisgarin in Azerbaijan; bol'sheglazyi puzanok or bigeye
shad, Sapozhnikovskii puzanok or Saposhnikovi shad, all in Russian].
Systematics
The lectotype of Clupea saposchnikowii from the Volga Delta
is in the Zoological Institute, St. Petersburg under ZISP 15921 (Berg,
1948-1949; Eschmeyer et al., 1996). The name is often spelt saposchnikovi,
in error, or with a single terminal "i"; Reshetnikov et
al. (1997) revert to the original spelling of the specific name.
Caspialosa caspia nigra Kisselevitsh, 1923, in part, from
the Caspian Sea opposite Dzambai is a synonym with a lectotype in the
Zoological Institute, St. Petersburg (ZISP 15938) (Kisselevitsh is
also transliterated Kiselevich and Kisselevitz). The material also
included specimens of Alosa braschnikowii (Whitehead, 1985;
Eschmeyer et al., 1996).
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. The upper
and lower head profiles are straight. The upper edge of the lower jaw
is straight. Total gill rakers 24-41, short (obviously shorter than
the gill filaments), and thick. Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-5, usually 4, unbranched rays and 12-15, mostly
13, branched rays, anal fin with 2-4, usually 3, unbranched rays and
15-21, mostly 18, branched rays. Lateral series scales 52-55.
Vertebrae 47-53. Pyloric caeca 36-59.
Sexual dimorphism
None reported.
Colour
Fish from the southern Caspian Sea are more intensively coloured
than those from the north. The back is violet with green sheen, the
flank has 4 dark stripes which merge with the dark on the back. There
is a spot posterior to the operculum, which may be absent, and there
is no series of spots.
Size
Reaches 36 cm total length and 650 g.
Distribution
Found mainly in the north Caspian Sea and the coast of Dagestan but entering Iranian waters.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
This species spends its whole life in the Caspian Sea and never
enters rivers. It favours colder water and is one of the first clupeid
species to migrate north in spring, principally along the western
coast. Large fish migrate first. Fish first approach the shore of
Azerbaijan in mid-March with a mass approach from late March to
mid-April. It is less frequently encountered in the southern part of
the Caspian Sea, overwintering in the central Caspian and only moving
south if winters are cold. A Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) has it at 400-600 m in the southern Caspian in cold winters but later
states it keeps at 15-32 m. Winter temperatures at which this species
is found are 6-7°C. Depths are 25-32 m in winter, more shallow in summer but below 9 m. Knipovich (1921)
reports this species in a depth range of 52-77 m in
Iranian waters. It tolerates a range of 3-25°C and spawns at salinities of
0.7-11.0‰, although preferring 4.0-7.5‰. The Caspian Sea Biodiversity
Database (from www.caspianenvironment.org) estimates a population of 1.1125 billion fish.
Age and growth
Life span is about 9 years and female lengths and weights exceed
those of males throughout life. On average, males weigh less than half
the weight of females since females carry a heavy egg load. Growth is
most intensive in the first two years of life and slows thereafter
(Chang, 1972). Males mature at age 2 and females at age 3.
Food
A rapacious fish which takes young herrings and kilka, Atherina and
even Benthophilus (Lönnberg, 1900b) as well as large
crustaceans such as mysids and gammarids. It is a cannibal. This shad overwinters and
feeds in the south Caspian Sea (Chang, 1972).
Reproduction
The spring spawning migration (end of April to end of May) enters the north Caspian Sea and
fish are mostly 15-25 cm in body length. Males mature at a younger age
than females as evidenced by fish 3-4 years old predominating among
females and fish 2-4 years old among males in the north Caspian catch.
Spawning takes place in May (peaking in the first 10 days) and most fish are returning for the second
time. Spawning temperatures are lower than in Alosa caspia,
being only 13-14°C although the peak is at 19-20°C.
Spawning occurs in il'mens, the sea where there is a freshwater
discharge such as near the Volga River mouth, and in the northeastern
sea. Females may spawn up to 6 times and males up to 5 times (Chang,
1972). Spawning takes place in shallow water at 1-6 m depths.
Fecundity is up to 318,852 eggs. The young migrate southwards.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
An important commercial species in the central and northern
Caspian, taken on their way to, and on, the spawning grounds. The
fishery in Azerbaijan during 1937 caught fish on average 17 cm long
and 62 g in weight, most fish being 2-3 years old. The Caspian catch
in the period 1936-1939 reached a peak of 8,800 t annually.
Fish are caught with beach seines, stationary nets and drift nets.
Conservation
Stocks in Iranian waters are reputed to be depleted. Kiabi et al.
(1999) consider this species to be data deficient in the south Caspian
Sea basin according to IUCN criteria. Criteria include numbers
unknown, range unknown, absent in other water bodies in Iran, absent
outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters and the stocks or taxa found
there need to be elucidated.
Sources
See under family heading.
Iranian material: CMNFI 1970-0531, 15, 49.9-108.7 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1970-0532, 1, 137.4 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 2, 78.8-80.2 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0581, 1, 102.1 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1979-0788, 3, 96.0-114.9 mm standard length, Mazandaran at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0136, 3, 107.3-127.6 mm standard length, Mazandaran, Fereydun Kenar River (36º41'N, 52º29'E);
CMNFI 1980-0157, 2, 96.6-101.1 mm standard length, Mazandaran, Gorgan River estuary (36º59'N, 53º59'30"E);
CMNFI 1980-0908, 1, 77.9 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E).
Comparative material: BM(NH) 1954.6.24:8-10, 3, 150.5-177.0 mm standard
length, Caspian Sea (no other locality data).
Alosa sphaerocephala
(Berg, 1913)
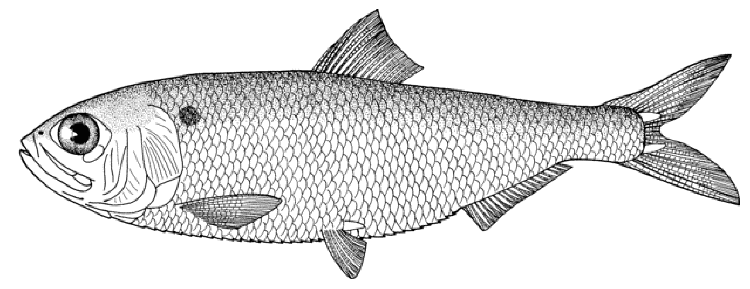
Common names
shagmahi-ye Agrakhan.
[kruglogolovyi puzanok or roundheaded shad, Agrakhanskii puzanok or Agrakhan shad, both in Russian].
Systematics
The holotype of Clupeonella sphaerocephala from Agrakhan
Bay, at Tyulenii Island, Turali in the northern part of the Caspian
Sea is in the Zoological Institute, St. Petersburg under ZISP 15928
with more than 30 paratypes (Eschmeyer et al., 1996).
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. The upper
and lower head profiles are obviously rounded. The upper edge of the
lower jaw is crescent-shaped. Total gill rakers 25-45, long (equal to
or longer than the gill filaments), and thin. Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-4, usually 4, unbranched rays and 13-15 branched
rays, anal fin with 3-4, usually 3, unbranched rays and 17-20 branched rays. Vertebrae 47-51.
Sexual dimorphism
None reported.
Colour
The back is dark with an olive tint, the tip of the snout is
occasionally black and the pectoral fins are dark. There is a black
spot behind the operculum and occasionally a row of such spots.
Size
Reaches 25 cm.
Distribution
Found in the Caspian Sea including Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
This species does not enter fresh waters. It is most common along
the eastern shore of the northern part of the sea in spring where
spawning occurs and along the northern shore of the northern part of
the sea in summer. Knipovich (1921) reports this species from Iranian
waters in a depth range of 52-77 m.
Age and growth
Unknown.
Food
Unknown, although presumably similar to other shads.
Reproduction
Spawning takes place in the northeastern Caspian from mid-May to
the end of June peaking at 18-20°C,
most frequently in a salinity of 8-11‰ and in depths around 3-8 m.
The young move south in late autumn, as late as November, the last
clupeids to leave this area. Fecundity is about 20,000 eggs.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
This species is caught only in small numbers.
Conservation
The status of this species is unknown.
Further work
This species is poorly known biologically and studies in Iranian
waters should be carried out on its life history.
Sources
Iranian material: None available.
Comparative material: BM(NH) 1954.6.24:11-13, 3,
145.6-162.1 mm standard length, Caspian Sea (no other locality data).
Alosa
volgensis
(Berg, 1913)
Recorded from Iranian waters by Kottelat and Freyhof (2007) but
presence needs confirmation by specimens.
Genus Clupeonella
Kessler, 1877
This genus is found in the Black and Caspian seas basins with 5 species, 3
of which are in the Caspian Sea and in Iranian waters.
Clupeonella species are distinguished from sympatric Alosa
species by smaller size, a small and toothless mouth, adipose eyelids
are small or rudimentary, no spots on the flank, no elongate scales
(ala) at the base of the caudal fin, no vomerine teeth, the lack of a
notch at the mid-line of the upper jaw, and by the last two anal fin
rays being elongated.
Species in this genus live entirely in the sea, or in fresh water, or
migrate between the two. Eggs are pelagic and have a large oil globule.
The general Farsi name for these fishes is كيلكا (= kilka or kelka, i.e.
"sprat", although sprat is erroneous according to Berg
(1948-1949) who uses tyulka for these fishes).
The three Clupeonella species have been fished in modern Iran since
December 1971 but the commercial catch did not exceed 15,000 tonnes. Earlier
catches date back only to 1939 with an annual catch of about 100 t in
1943-1949 exported in a marinated form to the Soviet Union (Alam, no date).
Curiously, the abundance of kilka has long been known as Kinneir
(1813) records "and herrings are in such abundance, that after a
storm, the shores of Ghilan and Mazanderaun are nearly
covered with them". Caddy (1984) refers to the kilka fisheries of
the Iranian Caspian by the scientific name Sprattus sprattus
but this is an error.
Caddy (1984) indicated that there were problems
in marketing and utilizing these fishes in Iran even though up to
50,000 t could be caught annually (200,000 t elsewhere in
the same article). Their best use was probably as food for predators
such as Sander lucioperca, Esox lucius and Salmo
caspius. A study by Razavi Sayad (1993b) suggested a ceiling of
100,000 t was possible. The Caspian Sea resources of kilka is
estimated at 800,000 t from which 340,000 t can be exploited
(Abzeeyan, Tehran, 6(8):IV, 1995).
The catch reached 51,000 t
in 1994 from none 10 years previously (Food and Agriculture
Organization, Fisheries Department, 1996) and was 36,000 t in
1997-1998 (IRNA, 31 March 1998) and 85,000 t in 1998-1999 (Fazli and
Roohi, 2002). The catch for the first 6
months of the Iranian year was 17,000 t, taken by 70 trawlers and
a 10% increase over the previous year (IRNA, 20 October 1998).
Fishermen in Gilan caught 50,000 t annually in the late 1990s (Tehran
Times, 5 September 1999). A reported catch of 56,000 t was
made in 1999-2000, a 13% increase over the previous year (IRNA,
27 March 2000). A later estimate expects the kilka catch to reach
66,000 t by the year 2000 (Abzeeyan, Tehran, 5(9):IV, 1995).
Fazli (2006a) records that kilka fishing ships discharge their catches at three
ports, Babolsar and Amirabad in Mazandaran and Anzali in Gilan. The catch
decreased from 28,000 t to 19,600 t in Mazandaran and from 57,000 to
42,600 t in Gilan from 1999 to 2000. The catch per unit effort also
decreased from 3900 kg to 2500 kg over the two years. Anchovy kilka dominated
the catch but the frequency fell from 85-90% to 76% of the catch and common
kilka sharply increased. Common kilka had been caught in spring and summer but
in 2000 they were taken in all months. The average length of anchovy kilka declined
from 96.3 mm in 1997 to 87.3 mm in 2000 and this was also reflected in the
age structure, 5+ and 6+ fish being rare. The presence of the ctenophore,
Mnemiopsis leidyi, was thought to be damaging stocks (Fazli and Roohi, 2002). Darvishi et al.
(200$) studied dietary overlap between the ctenophore and the anchovy kilka (see
below). Fazli (no date) studied kilka catches off Mazandaran in 1996-2000.
Fishing occurred at night and lasted 7.78-8.22 hours. The maximum catch at 42.8%
was taken in October, November and December with a minimum catch in June. The
least annual catch per vessel occurred in 1999-2000 (499,401 kg).
A study utilizing an echo-sounder and a pelagic trawler concludes
that the maximum biomasses for the three Clupeonella species in
the southern Caspian Sea are in winter (422,300 t) and autumn (326,900
t) while in summer and spring values are lower at 275,100 t and
260,800 t respectively. The population consists of 66.1% anchovy kilka (C.
engrauliformis), 18.9% bigeye kilka (C. grimmi) and 15% common kilka
(C. caspia)
(Iranian Fisheries Research and Training Organization Newsletter,
14:6, 1996). Note that later, the Iranian Fisheries Research and
Training Organization Newsletter (17:3, 1997) gives kilka biomass
in the southern Caspian Sea as winter 22,300 t, autumn 26,900 t,
summer 75,100 and spring 60,800 t, presumably lacking the initial
digit, and the percentages of kilka species in the biomass are also
wrong. This is corrected in a subsequent newsletter (Iranian
Fisheries Research and Training Organization Newsletter, Tehran
(18:43, 1997) but the corrected percentage biomasses are given as 66%
for C. engrauliformis, 19% for C. caspia (as C.
delicatula) and 15% for C. grimmi. It is unclear whether grimmi
or caspia is the second most important kilka species. Pourgholam
et al. (1996) give a stock assessment for kilkas in 1995-1995 using the
hydro-acoustic method.
C. engrauliformis dominates the catch in Iran at 91.8%,
followed by C. grimmi at 6.84% and by C. caspia
at only 1.35%. The 2+ and 3+ year classes account for 69.95% of C.
engrauliformis, 81.06% of C. grimmi and 80.88% of C.
caspia catches. Catch rates of kilka on the top ranking 17
fishing grounds of 56 studied range from 800 to 1200 kg per unit
effort per hour while traditional grounds have rates of 400-800 kg per
unit effort per hour. The kilka are caught by attraction to lights and netting
or pumping the catch into specially constructed ships. The kilka fishing
fleet of Iran expanded in the 1980s and 1990s. There were 30 active
vessels in Mazandaran in 1994, each with a capacity up to 30 tons (sic,
probably tonnes here and elsewhere for modern catches) (Abzeeyan, Tehran, 4(10):IV,
1994). The Mazandaran Kilka Cooperative Companies Union had 75 boats in 2000 (Tehran
Times, 31 December 2000). Gilan planned to construct 12 fish meal factories each with an
annual capacity of 1000 t and 10 kilka canneries also with 1000 ton
capacities (Abzeeyan, Tehran, 4(4):III, 1993). Catches off
Gilan alone from April 1994 to January 1995 increased 59% compared to
the same period in 1993-1994, exceeding 20,000 t (Abzeeyan,
Tehran, 6(1):II, 1995). The catch off Mazandaran from March 1994 to
March 1995 was 15,400 t, an increase of 10% over the previous
year. About 1000 t were processed for human consumption and the
rest for fishmeal production (Abzeeyan, Tehran, 6(2):V, 1995).
The total kilka catch for Iran has increased to 45,000 t
annually and efforts were being made to increase it to 110,000 t (Abzeeyan,
Tehran 4(5):IV, 1993). The catch in 1995 was 32,000 t with 64.7%
from Mazandaran and 35.3% from Gilan, with the maximum catch occurring
in April (Abzeeyan, Tehran, 7(6):II, 1996). Catches declined from 95,000
t in 1999 to 15,497 t in 2003 (Sayyad Bourani et al., 2008). Annual Soviet catches reached
37,000 t in 1956 but this declined to 300-1500 t by the end of the 1970s or
0.2-0.8% of all kinds of tyulka or kilka in the Caspian Sea. Turkmenistan
harvested 7660 and 8500 t in 1995 and 1996 although previously
almost 45,000 t valued at $22.5 million had been taken before
equipment deteriorated (http://bisnis.doc.gov/bisnis/isa/9805fish.htm,
downloaded 14 March 2000). Stocks remain large even though kilka are heavily fished.
Kilka are smoked, salted, canned in sauce and oil and marinated according to a traditional recipe and
seasoned with fruits, herbs and vegetables (Keivany and Nasrollahzadeh, 1990;
www.netiran.com/business.html, downloaded 31 October 2003). Moini and Koochekain (2003) give details of fish
sauce production from kilkas using traditional, microbial and enzymatic methods,
along with taste tests. Vacuum packaging of fresh, smoked and salted kilka has been investigated in
Iran (Annual Report, 1995-1996, Iranian Fisheries Research and
Training Organization, Tehran, p. 45-46, 1997) and studies on
processing kilkas as fish balls have also been carried out (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 40, 1996). Koochekian Sabour and Moini (2009)
describe investigations on using Iranian kilkas to produce a fermented fish
sauce for marketing in Southeast Asian countries. One company markets kilka in a clear
package which gives the product a bright and colourful appearance. Kilka have even been made into
crackers (Iranian Fisheries Research and Training Organization
Newsletter, Tehran, 18:6, 1997; Shojaei, 1998). Kilka have also been made
into oil as a by-product of the fish meal industry (Iranian Fisheries Research and
Training Organization Newsletter, 27:3, 2001). Omega-3 fatty acids have been
extracted from kilka oil under laboratory conditions (Salmani Joloudar et al.,
2009). M. Shivazad , H. John Mohammady, A. A. Yousef Hakimi and
H. Fazaely (http://iman.ut.ac.ir/news/agr.htm, downloaded 12 December 2004) discuss the use of Clupeonella engauliformis
as fish meal in animal nutrition and analyse the protein quality and Faeed et
al. (2006) studied spoilage in kilka meal from bacteria and fungi.
The Iranian Fisheries Research and Training Organization Newsletter (20:4,
1998) and Rezaei et al. (2003) report on methods of transporting kilka in cold water and
crushed ice to processing factories which were better than traditional methods.
Salmani et al. (2001) recommend chilled sea water for preservation for human consumption.
Motamedzadegan et al. (2009) found that partial hydrolysis of fish
myofibrillar proteins using papain improves its functionality. Motalebi et al. (2010) investigated the use of whey protein coating on quality
and shelf life of kilkas; it can enhance quality and increase frozen shelf life
in fish stored for up to 4 months. Khanedan et al. (2011) found that an
edible film of sodium alginate on dressed kilka increased shelf life. Valipour
Meri et al. (2011) showed that Bacilus licheniformis can decrease
significantly the aflotoxins in kilka fishmeal. Khoshkhoo et al. (2012)
examined protein and lipid changes in fish protein concentrate made from kilkas
over six months showing greater changes in Modified Atmosphere Packaging
compared to Vacuum Packing and less changes at lower storage temperatures.
The kilka fisheries are threatened by the comb
jelly, Mnemiopsis leidyi, which arrived in the Caspian in 1995 in the
ballast water of ships and spread through the entire sea by the year 2000, feeding
voraciously on zooplankton. It is now known as the "Caspian monster"
despite its small size of 5 cm (Muir, 2001). It doubles in size in one
day, reaches maturity in two weeks and then produces 8000 young each day (Muir,
2001). The fisheries collapsed by 50% in a few months, catches by one fisherman
falling from being 3-6 t a night to half a tonne. Ghadirneja (2003) reports
that C. engrauliformis originally dominated the kilka catch at
85-90% but has dropped to 55% through the impact of the comb jelly which has up
to 2285 individuals per cubic metre in the southwest Caspian Sea. Fazli
et al. (2009) describe a multi-species approach for stock management,
allowing for the decline of C. engrauliformis and increase in C.
caspia in Iranian waters through competition with the ctenophore. The fisheries may recover somewhat after
the comb jelly population collapses (Tidwell, 2001b) or if a predator, Beroe ovata,
is introduced and can survive in the less saline waters of the Caspian Sea
(Muir, 2001). Studies indicate it can survive the brackish Caspian Sea water,
feed on the comb jelly and not feed on other plankton (Iranian Fisheries Research Organization
Newsletter, 36:35, 2003). The following catch records for the total kilka catch in
Mazandaran in tonnes is courtesy of F. Darvishi (pers. comm., 2003) and shows
the drastic decline caused by the ctenophore, as well as monthly variations in
catches:-
| Months/Years |
1998 (1377) |
1999 (1378) |
2000 (1379) |
2001 (1380) |
2002 (1381) |
Mean |
| March-April (Farvardin) |
2848 |
2703 |
4644 |
1217 |
876 |
2458 |
| April-May (Ordibehesht) |
1116 |
607 |
972 |
1422 |
195 |
862 |
| May-June (Khordad) |
370 |
763 |
1819 |
125 |
158 |
647 |
| June-July
(Tir) |
1392 |
919 |
194 |
425 |
444 |
675 |
| July-August
(Mordad) |
2152 |
2306 |
433 |
614 |
249 |
1151 |
| August-September
(Shahrivar) |
3117 |
2010 |
581 |
528 |
336 |
1314 |
| September-October
(Mehr) |
3103 |
6184 |
1785 |
432 |
575 |
2416 |
| October-November
(Aban) |
4120 |
3468 |
2305 |
3051 |
1196 |
2828 |
| November-December
(Azar) |
3835 |
3410 |
2655 |
993 |
- |
2723*
(2179) |
| December-January
(Dey) |
2754 |
1735 |
620 |
1082 |
- |
1548*
(1238) |
| January-February
(Bahman) |
3968 |
1262 |
2146 |
1586 |
- |
2241*
(1792) |
| February-March
(Esfand) |
2815 |
1667 |
1192 |
1903 |
- |
1894*
(1515) |
| Total |
31,590 |
28,034 |
19,648 |
13,378 |
4029 |
|
* = averaged over 4 and (5) years.
The species composition of kilkas changed after the introduction of the comb jelly comparing the year 2000
and before with the year 2002 - the common kilka changed from about 1-5% to
about 30%, the bigeye from about 10-15% to 0/2% and the anchovy kilka from about
85-90% to about 70% (Iranian Fisheries Research Organization Newsletter, 36:2, 2003). The catch per unit effort
(catch per vessel per fishing night) fell from 4 t to 1 t.
The catch during 1997-1999 of anchovy kilka fell from 51,300 t to 491 t and
bigeye kilka from 7600 t to 309 t while common kilka rose from 1500 t to 24,600
t (Iranian Fisheries Research Organization Newsletter, 65:4, 2011).
Parafkandeh Haghighi and Kaymaram (2012) found, for the years 2006-2007, that
the common kilka was the dominant species (89.7%) while the anchovy kilka was at
only 8.7% after previously being the dominant species. This was attributed to
the comb jelly which occupied the anchovy kilka habitat at depths greater than
50 m. The total catch of kilkas fell from 95,000 t in 1999 to less than 20,000 t
in 2007. The fishery moved to areas with depths less than 50 m, the main reason
for the change in species composition.
In 2004, more than 200 fishing boats had been forced to stop operations. The kilka stock has been reduced from 400,000
t to 80,000 t over the past 4 years and the catch fell by 34,000 t
(www.iranmania.com, downloaded 4 October 2004). See also the section on the Caspian Sea basin in the Introduction.
Mamedov (2006) gives details of the biology and decline of kilkas in Azerbaijan
waters.
The Caspian seal was once a major predator on kilkas but the
number of seals has declined on the Kazakhstan and Iranian coasts from 300,000
to 5000 in recent years through DDT pollution, viral infections and food shortages (Hashemi, 2001).
An account on the biology and identification of Caspian kilka in Farsi is given by Emadi (1991)
and Fazli (1990), Fazli and Besharat (1998) and Poorgholam et al.
(1996) give accounts of biology and catches in Iran in Farsi.
Clupeonella caspia
Svetovidov, 1941
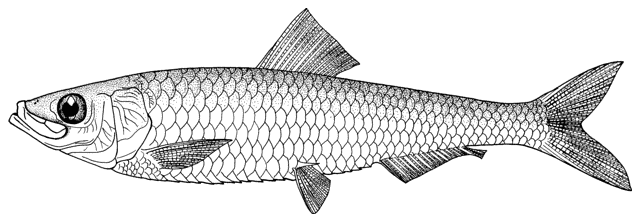
Common names
rizeh keraye (= tiny ?), rizeh kuli, kilka-ye ma'muli or kilka-e-maamooli (= common shad).
[xazar kilkasi in Azerbaijanian; adaty kulke balyk in Turkmenian; Kaspiiskaya tyul'ka or kil'ka (i.e.
Caspian tyulka or kilka), tyulka, obyknovennaya tyul'ka (i.e. common tyulka), all in Russian; common kilka, common Caspian kilka, sardelle,
Caspian sprat, Black Sea sprat].
Systematics
Formerly identified as Clupea cultriventris, originally described from the
northern shore of the Black Sea. Clupea delicatula Nordmann, 1840, described from Odessa
market on the Black Sea, is a synonym of C. cultriventris and a lectotype is in the
Zoological Museum. St. Petersburg under ZISP 2254 with paralectotypes
also under ZISP 2254, as designated by Svetovidov (1952). Clupeonella
delicatula caspia Svetovidov, 1941 was considered to be a synonym and was described
as from the
"Caspian Sea, where it is met with almost everywhere, from very
saline parts (Kaydak Bay) to quite fresh. Enters the mouths of the
Volga and the Ural rivers, ascending sometimes very far
upstream". The holotype is from the Volga Delta and is under ZISP
15883 (Svetovidov, 1952). Kottelat and Freyhof (2007) consider this subspecies
to be a a distinct species found in the Caspian Sea with cultriventris
restricted to the Black Sea. Reshetnikov et al. (1997) consider
recognition of this subspecies as questionable.
The Caspian Sea taxon, Clupeonella caspia,
has a lectotype, 152
mm long, designated by Svetovidov (1952) in the Zoological
Institute, St. Petersburg (ZISP 15883).
Clupea cultriventris is spelled cultiventris
in some parts of Eschmeyer et al. (1996), apparently in error.
Three syntypes of Clupea cultriventris may be in the Muséum
National d'Histoire Naturelle, Paris under MNHN 3681 (Svetovidov,
1952; Eschmeyer et al., 1996).
Clupea cultriventris var. tscharchalensis Borodin,
1896 from Lake Charkhal in the Ural River basin is variously listed as
a variety, morpha or a distinct species
(see Svetovidov (1952) and Kottelat and Freyhof (2007)).
mtDNA studies of fish from Mazandaran and from Gilan showed statistically
significant differences in haplotype frequencies, indicating genetically
different populations (Laloei et al., 2006).
Key characters
This species has a moderately deep body (21-27% of standard
length), a short and wide head (interorbital width 17.5% or more of
head length), a sharply keeled belly, and pointed pectoral fin tips.
The Caspian subspecies is distinguished from the type subspecies of
the Black Sea by having shorter pectoral (15.5-19.0% of standard length) and pelvic fins
(8.5-12.5% of standard length), although ranges overlap, a shallower
body, and a shallower and shorter head. It also grows faster and is
more fatty than the Black Sea subspecies.
Morphology
The dorsal fin has 3-4 unbranched rays, usually 3, followed by
11-14 branched rays and the anal fin has 1-3 unbranched rays, usually
3, and 14-19 branched rays. Scales in lateral series 42-55. There are
24-30 belly scutes and 41-62 (rarely to 64), usually 51 or more, gill
rakers. Vertebrae 40-44 (rarely to 45) compared to 44-47 in the
anchovy kilka and 46-48 in the bigeye kilka, probably as a result of
higher water temperatures during development compared to other kilka
species (Prikhod'ko, 1979b).
Sexual dimorphism
Sexual dimorphism is only evident during egg development when the
belly of females is swollen.
Colour
The back is blue-green or light-green, the flanks silvery and the
belly silvery-white or golden-yellow. Fins are hyaline except the dorsal fin which has
a central dark but faint stripe and the caudal fin which is darkish at
the base. The iris is black.
Size
Reaches 14.5 cm standard length and 19 g.
Distribution
Found in the Black and Caspian seas, tributary rivers and some
adjacent lakes. In Iran, it is reported from sea and also the confluence of the Pasikhan and Pir
Bazar rivers of the Anzali Mordab, the Anzali Mordab and its outlets
by Holčík and Oláh (1992) and from the Safid River and Anzali Talab (= Mordab) by Abbasi
et al. (1999).
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
The habitat of this species in the Caspian Sea is the coastal zone
of the sea at depths less than 100 m, more usually less than 50-70 m, over a wide range of
temperatures (2.6-27.6°C for adults, higher for larvae, and possibly lower temperatures since
they are found under ice and probably over 28°C
according to some reports), and in fresh and hypersaline waters (to 36‰).
The young can develop in water at 16‰. Southern populations live in
a more saline habitat than northern and central Caspian populations
which are mostly in fresh water. This tyulka may not migrate far but
does move between summer-winter feeding and spring-early summer
spawning grounds. Large schools are found 0.5-2.0 km from shore at
depths of 20-25 m on the eastern coast of the Caspian Sea, descending
deeper if water temperatures rise and coming up to about 8 m in autumn
as temperatures fall. In winter this species is found at about 30-40 m
deep where the temperature range is 7-10°C,
warmer than surface waters. Larvae and young remain in shallow coastal
areas. Knipovich (1921) reports a fish from a depth range of 235-300 m
in Iranian waters but populations at these depths are small (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996). The Caspian Sea Biodiversity Database (from www.caspianenvironment.org)
states that the largest concentrations are found at 3-7‰ with most
intensive spawning at 2-4‰.
It is the most widely distributed kilka and with the other kilka
species the most abundant fish in the Caspian Sea (Prikhod'ko, 1979b).
Large schools can be found by day but these disperse at night. It
overwinters in the southern Caspian Sea and some individuals move
north to spawn and feed in April. The Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) estimates the population to number 224 billion fish, with 96 billion fish
in the south Caspian. The south and north Caspian Sea stocks are about equal in
number after a decline in copepod biomass in the north. The relative frequency
of this species compared to other kilkas increased after the invasion of
Mnemiopsis leidyi, by more than 10% (Fazli, 2006b; Fazli et al., 2006).
Age and growth
Osipov and Kiyashko (2008) found that using otoliths gave more reliable
estimates than using scales for ageing. The Caspian subspecies grows faster than the Black Sea
subspecies. Together with the sturgeons, this species comprises 82.1%
of the fish biomass in the Caspian Sea. Condition in this species is better in winter because of the
summer-autumn feeding period after spring spawning compared to C.
engrauliformis in the Big Kizil-Agach (= Bol'shoy Kyzylagach or
Imeni Kirova) Bay of Azerbaijan (Badalov, 1972). Local populations
have differing growth regimes depending on the productivity of these
areas (Prikhod'ko, 1979b) and there are great variations on a yearly
basis too. Southern populations grow faster than northern ones in
their first year. Females grow somewhat faster than males (9.0 g
versus 7.3 g average weight along the Dagestan coast for example), and
life span is about 6 years. This species is mature there at 1 year and
average life span is about 3 years.
Females dominate the population in
Iran and sexual maturity is attained usually at age 2 and 2-4 year
olds dominate catches but life span is up to 8 years (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996; Abtahi et al., 2002). Fazli (2006b) found age classes 0+ to 5+ in
Iranian waters with 0+ to 3+ making up 95% of the fish in 1997-1999. In 2000,
age classes 0+ and 1+ were reduced in numbers and 2+ to 4+ fish comprised
93.8%. Abtahi et al. (2004) examined fish from the conical net and
light catch at Babolsar and found average fork lengths were 69.82 mm, 83.56 mm,
88.38 mm and 88.43 mm while weights were 2.2 g, 4.18 g, 4.77 g and 5.06 g for
fishes at maturity stages I, II, II and IV. Fazli et al. (2007) studied
this species from 1995 to 2004 in Iranian waters, sampled at landing sites at Amirabad and Babolsar in Mazandaran and Anzali in Gilan. Growth parameters were
L∞ = 132 mm, K = 0.259/yr. t0 = -1.285/yr. The
instantaneous coefficient of natural mortality was 0.506/yr, the instantaneous
coefficient of total mortality (Z) was 1.62/yr and the instantaneous coefficient
of fishing mortality varied over 10 years from 0.125/yr to 1.487/yr. Annual
survival rate (S) was 0.200/yr. Age at first capture was 2.8 years. The von
Bertalanffy growth equation was Lt = 132 (1-e-0.259(t +1.285)).
Ages ranged from 1 to 7 years with age groups 2, 3 and 4 dominating at different
periods. Mean fork lengths were 59.3, 77.5, 87.4, 97.2, 104.5, 111.9 and 116.8
mm. Females dominated in each month except April, averaging 0.47:1, possibly due
to differing attraction to lights used in the fishery. Biomass increased from
16,000 mt in 1995 to more than 41,000 mt in 2002, declining to less than 28,000
mt in 2004. The increase was simultaneous with a sharp decline in anchovy kilka,
changes in zooplankton composition and abundance, and especially an increase in
zooplankton species favoured by this kilka. Currently this kilka is overfished.
Karimzadeh et al. (2010) and Karimzadeh (2011) examined fish from the Babolsar region off
Mazandaran and calculated growth parameters as L∞ = 143.5 mm, K = 0.30/yr-1
and t0 = -1.02/yr, instantaneous coefficient of natural
mortality was 0.671 yr-1, fishing mortality was 0.849 yr-1, and the current exploitation rate was
estimated as 0.55 and this species is now overfished.
Aliasghari et al. (2012) examined Iranian fish in 2010 and found a
length-weight relationship of W = 0.000001FL2.92, L∞
= 128.7 mm, K = 0.41/year, t0 = -0.59/year, survival rate =
0.239/year, natural mortality = 0.448/year, fishing mortality 0.983/year,
exploitation rate 0.687 (indicating overfishing), age groups 1-6 years, average
age 3.27 years, 3-year-old fish were the largest age group (45.24% of the
catch), and males were dominant (1:0.779). Mean length and weight of the
population increased but mean age decreased in recent years.
Food
Plankton is the main food and copepods predominate but diet also
includes Cladocera, Balanus larvae and clam larvae. The
dominant food item is the copepod Eurytemora grimmi,
particularly in winter when plankton biomass is lowered in the
Bol'shoy Kyzylagach Bay of Azerbaijan. The food of the common kilka is
more varied than the other kilka species simply because of its habitat
in shallow coastal areas (Badalov, 1972; Prikhod'ko, 1979b). Older
fish take larger and faster crustaceans and consume less food in
proportion to body size as they grow. The most intensive feeding is in
summer and autumn, decreasing in winter and during reproduction. Food
is taken during the day. Roushan Tabari et al. (2009) examined fish from
a fishing vessel of Mazandaran and found highest feeding activity in April with
280±153 prey items per fish weighing 2.9±1.6 mg. Balanus nauplii and
cypris larvae comprised 93% and Acartia 7% at this time with increasing
spring temperatures and reproduction, but the copepod Acartia biomass
dominated from October to February.
Reproduction
Spawning occurs in January-February in the southern Caspian, later
in the north, mainly in depths less than 10 m and where salinity is
low to average for the Caspian Sea (Badalov, 1972; Prikhod'ko, 1979b).
The largest southern Caspian population spawns near the mouths of the
Volga and Ural rivers (Kozlovsky in Hoestlandt, 1991). Spawning is
most intensive at 11°C, but occurs at 10-20°C.
Spawning is intermittent and lasts from mid-April to July. Peak spawning in
Iranian waters of Mazandaran Province is April-May with an average fecundity of
28,240 eggs (Abtahi et al., 2002). Fazli (2006b) recorded mass
spawning in Iranian waters in April, continuing on until August. Eggs are
released in water 0.5-9.0 m deep at a salinity range of 0.02-15‰,
perhaps as high as 29.15‰. Fecundity reaches 60,000 eggs and egg
diameter 1 mm, 0.48-1.46 mm for fertilised eggs. Relative fertility is
4-13 times greater than in Alosa species. Holčík and Oláh (1992)
consider that it may spawn in rivers entering the Anzali Mordab. The studies of Fazli et al. (2006; 2007) showed that reproduction started in March, peaked in
May and finished at the end of August. Half the females were mature at 84.3 mm
fork length. Aliasghari et al. (2012)
found from gonadosomatic indices and sexual maturity stages that spawning in
Iran began in February and peaked in May and June.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean parasites Pseudopentagramma symmetrica (probably
Pronoprymna ventricosa after Youssefi et al. (2010)) and Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum, metacercariae of a Bucephalus
species, and larvae of a Contracaecum and an Anisakis
species (Iranian Fisheries Research and Training Organization
Newsletter, 11:4-5, 1996; Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 28, 1997; Shamsi
and Dalimi, 1996; Shamsi et al., 1998).
Ghayoumi et al. (2009) found that fish from Babol Sar harbour contained
the intestinal helminths Corynosoma strumosum, Pronoprymna ventricosa,
Contracaecum sp. larvae and Raphidascaris sp. larvae, diet being
the main factor affecting diversity of the parasites. Varshoie et al. (2010) record the helminths Pseudopentagramma
symmetrica (see above), Bunocotyle cingulata and Mazocreas alosae in this
species from Iranian waters.
Clupeonella species are an important food fish for sturgeons
(59.4% by weight of Acipenser stellatus diet in the Middle
Caspian), Sander, herrings (Clupeidae) and the Caspian seal (Badalov,
1972; Krylov, 1984) as well as Salmo caspius and Stenodus
leucichthys (Kosarev and Yablonskaya, 1994).
Economic importance
It is caught by attraction to underwater electrical lights (Prikhod'ko,
1979b). The other subspecies is also of major importance in the Sea
of Azov. The Caspian subspecies is caught in school seines in spring
and purse seines in summer. In Iranian waters this species formed only
a small proportion (1.35%) of the total kilka catch in a study by
Razavi Sayad (1993b) and Fazli (2006b) gives values of 1.34%, 2.5% and 5.5% for
the years 1990-91, 1997-98 and 1998-99 respectively. However, as the anchovy kilka catch declined, this species increased from 13.7% of the total catch in
1999 to 48.9% in 2003 (Sayyad Bourani et al., 2008).
Naseri et al. (2010) studied lipid changes in canned kilka after
long-term storage. Motalebi and Seyfazadeh (2011) used an edible whey protein
coating on frozen common kilka and this helped retain a good quality in the
product.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and as bait.
Conservation
Stocks on the Iranian coast are said to have been depleted but its
ecological specialisation on zooplankton means there is comparatively
little competition with other fishes. It is probably not in any
immediate danger. Kiabi et al. (1999) consider this species to
be of least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include commercial fishing, abundant in numbers,
widespread range (75% of water bodies), absent in other water bodies
in Iran, and present outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Counts are based in part on Svetovidov (1945a). See also under family heading.
Iranian material: CMNFI 1970-0531, 14, 78.0-88.6 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1980-0146, 7, 79.9-96.2 mm standard length, Mazandaran, Gorgan Bay at Ashuradeh-ye Kuchak (36º50'N, 53º56'E);
CMNFI 1993-0146, 3, 80.2-98.2 mm standard length, Mazandaran, Gorgan Bay (no other locality data);
CMNFI 1993-0167, 1, 96.6 mm standard length, Mazandaran, Caspian Sea, 10 km offshore (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 3, 84.9-88.0 mm standard length, Mazandaran, Caspian Sea, 10 km offshore (ca. 36º49'N, ca. 52º39'E).
Clupeonella engrauliformis
(Borodin, 1904)

Common names
rizeh keraye (= tiny ?), kilka-ye anchovy or kilka-e-anchovi.
[ancousabanzar kilka in Azerbaijanian; ancous sekilli kulke balyk in
Turkmenian; anchousovidnaya tyul'ka or
anchovy-like tyulka, sardelle or sardel'ka, "sardinka" but
incorrectly, all in Russian; anchovy kilka, anchovy sprat].
Systematics
No major synonyms. Originally described from Buinak, central part
of the Caspian Sea. The lectotype is in the Zoological Institute, St.
Petersburg (ZISP 13860) with paralectotypes as established by
Svetovidov (1952) (Eschmeyer et al., 1996). Eschmeyer et al.
(1996) give the date as 1906 but Reshetnikov et al. (1997) give 1904.
Key characters
This species has a slender body (16-19% of standard length), a
short and wide head (interorbital width 16-18.5% of head length), a
rounded belly, and pointed pectoral fin tips.
Morphology
Dorsal fin with 3 unbranched and 12-14 branched rays, anal fin with
3 unbranched and 15-19 branched rays. Scales in lateral series 45-49.
Vertebrae 44-47, rarely to 48 compared to 41-44 in the common kilka (C.
caspia). Gill rakers number 56-67. Belly scutes 23-31.
Sexual dimorphism
None reported.
Colour
The back and head are dark blue with violet, green or olive tints. These colours become brighter or turn black in dead fish.
The fins are hyaline except the caudal fin which has a black base and the dorsal
fin which has a central dark stripe.
Size
Attains 15.5 cm standard length.
Distribution
Found in the central and southern Caspian Sea, and in Iranian waters the southeast Caspian Sea, southwest Caspian Sea and
the south-central Caspian Sea (Kiabi et al., 1999) as well as the Anzali Mordab,
Babol Sar Beach and Gorgan
Bay (Armantrout, 1980). Abdoli and Naderi (2009) list it as from the southwest,
southeast and south-central Caspian Sea, the Anzali Talab and Gorgan Bay in
Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
The anchovy kilka, along with other kilkas, is the most abundant
fish in the Caspian Sea forming large concentrations in the central
and southern Caspian wherever water depth exceeds 30 m. The anchovy kilka is
estimated to be the most numerous kilka at about 77% (Ivanov and Katunin, 2001;
Daskalov and Mamedov, 2007). It is generally found in the upper water layers but may descend
to 120 m. Nearshore areas, inlets and water of a salinity below 8‰ are
avoided. They can tolerate a salinity range of 8-14‰ but the main part of the
population is found at 10-12‰ (Fazli et al., 2007). Overwintering takes place in the southern Caspian and the
southern part of the central Caspian Sea at 8.5-9.0°C and up to 13.5°C.
Schools extend their range into the central and northern Caspian in
spring to feed (Prikhod'ko, 1979b). This species has a hibernation
period in the south Caspian Sea, a spring migration of part of the
population to the central Caspian, a feeding period in the central and
south Caspian and an autumn prespawning migration to the south Caspian
(Sedov and Rychagova, 1983).
In Iran larvae are found mostly in surface layers at 5-20 m while
adults are found in deeper zones. males dominate in winter while
females dominate in other seasons. The maximum juvenile density (fish
<75 mm), comprising 36% of the population, is seen in the summer (Iranian
Fisheries Research and Training Organization Newsletter, 20:7,
1998). Jolodar and Abdoli (2004) state it is most abundant at 100-150 m.
Age and growth
Abundance of young anchovy kilka, and hence future year-class
strengths, depends on water temperature in autumn (October-November).
Falling water temperatures, in the eastern Caspian for example, are
caused by upwelling which brings nutrients to surface waters and
promotes growth of plankton on which the kilka larvae feed (Prikhod'ko,
1979a). Females are somewhat larger than males in the spawning areas.
Sexual maturity is attained usually at age 2 and 2-4 year olds
dominate catches but life span is up to 8 years (Iranian Fisheries
Research and Training Organization Newsletter, 14:6, 1996). This
species shows the fastest rate of growth in the genus. Of the 8 age
classes, 0+, 1+, 2+ and 3+ form 99.91% of the whole population (Iranian
Fisheries Research and Training Organization Newsletter, 20:7,
1998). The same study showed that 18.6% of the population matures in
the first year of life while 81% matures in the second. The mean age
in coastal areas is 2.9 years, slightly higher than that in deep zones
below 200 m where 0+ fish are more abundant.
The Caspian Sea Biodiversity Database (from www.caspianenvironment.org)
gives a population of up to 293 billion fish in the Caspian Sea.
Fazli et al. (2007) and Sayyad Bourani et al. (2008) studied these kilkas from catches with conical
liftnets carrying underwater lights in the fisheries of Gilan and Mazandaran in
the 1995-2004 period. Fish were aged using the sagittal otoliths. Length and
weight ranges were 40-140 mm and 0.4-18.4 g with averages of 94.0 mm and 5.7 g
(89.2-100.4 mm from 1999 to 2003 in Sayyad Bourani et al., 2008).
The age range was 1-7 years (rarely to 8+ years in Parafkandeh Haghighi and
Kaymaram (2012)). The dominant age group varied from age 2 to age 4,
making up 40.6% to 57.7% of the catch (Fazli et al., 2007) or 5+ years
with 4+-5+ making up 84.6% for 1999-2003 (Sayyad Bourani et al., 2008). Growth was high for the first year of
life and then gradually decreased. The von Bertalanffy growth equation was Lt
= 148(1-e-0.238(t+1.340)) (Fazli et al., 2007, and following
data). The sex ratio varied with season and was
significantly different from equal at male:female = 0.78:1 for adults. Females
were more abundant from January to June and males predominated from September to
November. Condition factors differed significantly between years, increasing
from 1995 to 1996, being lowest in 1998 and then increasing to 2004, and between
months, being lowest in January and February and then increasing in March. 50%
of fish were mature at 84.5 mm fork length. Annual survival rate was estimated
at 0.32, the instantaneous coefficient of total mortality (Z) was 1.14/year,
natural mortality was 0.473/year. Age at first capture was estimated as 2.92
years. The total biomass declined from 186,000 t in 1996 to less than 12,000 t
in 2004 and the exploitation rate for 1995-2004 varied between 0.340 and 0.815.
Sayyad Bourani et al. (2008) give a K value of 0.598/year and a L∞
of 110.13 mm. Natural, fishing and total mortality coefficients were 0.69, 0.31
and 1 per year respectively and the sex ratio was female:male = 68.2-31.8. These
latter results for the 1999-2003 period show how value scan change when subsets
of data are used. Fatemi et al. (2009) examined fish taken from
commercial vessels in 2007 using lift nets and lights. Age structure ranged from
2 to 7 years and was dominated by the third year class (38.6%). Back-calculation
methods were validated using otoliths to determine lengths. Karimzadeh et al. (2010) examined fish from the
Babolsar region off Mazandaran and calculated growth parameters as L∞ = 151.9 mm, K = 0.28/yr-1
and t0 = -1.12/yr, instantaneous coefficient of natural mortality was
0.633/yr-1 and the current exploitation rate was estimated as 0.41.
Janbaz et al. (2012) give values for Iran from 2005 to 2007 of K = 0.375/year, L∞
131.7 mm, instantaneous coefficient of natural mortality was 0.49/year, fishing
mortality was 0.51/year and total mortality 1.0/year, and exploitation rate was 0.51.
Abundance declined over the three years from 18.8% to 8.5% and to 6%, catch per
unit effort declined from 0.3 to 0.1 t, and the condition factor declined.
Overfishing and the competitive ctenophore were the main causes of the decline.
Food
Plankton is the main food and copepods predominate but diet also
includes Cladocera, Balanus larvae and clam larvae. The
dominant food item is the copepod Eurytemora grimmi,
particularly in winter when plankton biomass is lowered (Badalov,
1972). It can make up over 70% of its food. This copepod is more
characteristic of the diet of this kilka compared to the other two
species and the daily vertical migrations and seasonal movements of
the copepod are mirrored by the kilka. The most abundant fish species
in the Caspian depends on the most abundant member of the crustacean
zooplankton (Prikhod'ko, 1979b). This species feeds in winter, unlike Clupeonella
caspia. Bankehsaz (1996) surveys the fluctuation in fat
content of this species through the year. Intensive feeding begins in
spring as a preparation for spawning (Sedov and Rychagova, 1983).
Spawning males show a positive response to light and so feed during
the spawning season, while females do not. F. Darvishi (pers. comm., 2003) has
demonstrated that the this species has a similar feeding niche as the exotic
ctenophore Mnemiopsis leidyi and Esmaili Sari et al. (2002)
determined that there is a similar diet in Iranian waters suggesting that a
decline in stocks of the fish is the result of competition.
Darvishi et al. (2004) studied catches of the anchovy kilka and the
ctenophore in the southern Caspian Sea from August 2001 to October 2002. Dietary
overlap was >89 in Babolsar samples and >84 in Nowshahr samples using the
Schoener Index (presumably 0.89 and 0.84 where 0 is no dietary overlap and 1 is
an identical diet). The ctenophore was also feeding on fish eggs but the effect
of this was less than competition for food.
Reproduction
Spawning ends in late autumn and winter food requirements are
higher than in spring-spawning C. caspia (Badalov,
1972). Areas for spawning in this species are extensive. Spawning is
most intensive in July when temperatures are 13-24°C
and salinity 8-13‰ although the Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) gives peak spawning (70%) as in October-November. Fazli (2006a) gives
spawning in Iran as spring and autumn but mass spawning takes place in in
autumn. Spawning takes place in the central and southern
Caspian along both eastern and western shores both in coastal regions
and the open sea from late April to November. Mass spawning takes
place at depths of 50-200 m and as a result eggs and larvae are
carried over a wide area by the Caspian gyral current at these depths
(Prikhod'ko, 1979b). Young hatch mainly in autumn and reach 4.5-8.0 cm
at an age of 8-10 months (Prikhod'ko, 1979a). Eggs are up to 1.82 mm
in diameter and fecundity reaches 39,900 eggs.
In Iran, 80% of the population spawn in autumn and the remainder in
spring. Accordingly the fishery should be closed in October and
November (Iranian Fisheries Research and Training Organization
Newsletter, 19:5, 1998). The subsequent Iranian Fisheries
Research and Training Organization Newsletter (20:7, 1998) states
that 89% of the population spawns in autumn with September, at 68.3%, the major month.
Fazli et al. (2007) found reproduction to start in June, peaking in
October and then declining.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean trematode parasites Pseudopentagramma symmetrica (probably
Pronoprymna ventricosa after Youssefi et al. (2010)) and Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum and
larvae of the nematode Contracaecum sp. (Iranian Fisheries
Research and Training Organization Newsletter, 11:4-5, 1996;
Shamsi et al., 1996; Annual Report, 1995-1996, Iranian Fisheries
Research and Training Organization, Tehran, p. 28, 1997; Shamsi
and Dalimi, 1996; Shamsi et al., 1998). Clupeonella species are an
important food fish for sturgeons (59.4% by weight of sevryuga diet in
the Middle Caspian), Sander (Percidae) and herrings and the
Caspian seal (Badalov, 1972; Krylov, 1984) as well as other fishes. Ghayoumi
et al. (2009) found that fish from Babol Sar harbour contained the
intestinal helminths Corynosoma strumosum, Pronoprymna ventricosa,
Contracaecum sp. larvae and Raphidascaris sp. larvae, diet
being the main factor affecting diversity of the parasites. Varshoie
et al. (2010) record the helminths Pseudopentagramma symmetrica (see
above),
Bunocotyle cingulata and Mazocreas alosae in this species from
Iranian waters.
Economic importance
This species forms 80-90% of the catches of kilkas in former Soviet
waters (Sedov and Rychagova, 1983) and, as noted above, 91.8% of
catches in an Iranian study (Razavi Sayad, 1993b; Rezaei et al., 2003). High catches are
related to the larger spawning and foraging range of this species
compared to other kilkas and to its habitat in the Caspian gyre, an
area of increased biological productivity (Prikhod'ko, 1979b). It is
caught in former Soviet waters by attraction to underwater electrical
lights attached to the middle of the mouth of a fine-mesh conical net
or the sides of a fish pump (Ben-Yami, 1976). Fishing is suspended at full moons
as the fish are dispersed (Saheli, 1999). Both large and small
individuals are taken by these non-selective methods (Prikhod'ko,
1981). Incidental catches include Mugilidae (common), and Alosa
spp., Atherinidae and the cyprinid Pelecus cultratus (all
occasional) (Ben-Yami, 1976).
It is regarded as a valuable and cheap food resource in Iran where
it is canned, made into sausages and surimi, and processed as fish meal (Shamsi et
al., 1996; Moeini, 2002; Shabanpour et al., 2002,,2006). The catch per unit effort for funnel nets and midwater
trawls is 2321 and 1014 respectively (Iranian Fisheries Research
and Training Organization Newsletter, 20:7, 1998).
Various studies on its preparation and storage as food have been carried out,
e.g. Rezaei et al. (2002; 2003; Moeini et al., 2009).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and as bait.
Conservation
Prikhod'ko (1981) recommends fishing in deeper waters where larger
fish are concentrated to avoid an excessive take of young fish which
favour the upper water layers. Stocks in the southern Caspian Sea are
said to be depleted. Kiabi et al. (1999) consider this species
to be of least concern in the south Caspian Sea basin according to
IUCN criteria. Criteria include commercial fishing, abundant in
numbers, widespread range (75% of water bodies), absent in other water
bodies in Iran, absent outside the Caspian Sea basin. Daskalov and Mamedov
(2007) studied commercial catch data in the Caspian Sea generally and found a
period of high catches from 1991 to 2000 with high spawning-stock biomass and
relatively good recruitment. Catches peaked at 271,400 t, fishing mortality
reached 1.8y-1 in 1999 and overfishing occurred. From 2001 to 2004,
the stock collapsed, recruitment failed in 2001 and catches fell to 54,300 t in
2005. This was attributed to the spread of the ctenophore Mnemiopsis leidyi,
with contributions from overfishing. Fazli et al. (2007) also concur that
both overfishing and the invasive ctenophore caused the collapse of stocks. The
catch in Iran declined from 71% of the total kilka catch in 1999 to 52.5% in
2003 (Sayyad Bourani et al., 2008).
Janbaz et al. (2012) give figures for the collapse in Iran from 4250 t in
2005 to 924 t in 2007.
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Iranian material: CMNFI 1993-0167, 1, 99.5 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 4, 89.3-107.6 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E).
Clupeonella grimmi
Kessler, 1877

Common names
kilka-ye cheshmdorosht (= bigeye kilka).
[irikoz kilka in Azerbaijan; sardelle or sardel'ka, bol'sheglazaya
tyul'ka or bigeye tyulka, bol'sheglazaya kil'ka or bigeye kilka, all
in Russian; southern Caspian sprat].
Systematics
Clupeonella Grimmi was originally described from the central
part of the Caspian Sea. The lectotype is in the Zoological Institute,
St. Petersburg under ZISP 10934 as designated by Svetovidov (1952).
Harengula macrophthalma Knipovich, 1921 is a synonym. Four
syntypes are in the Natural History Museum, London under BM(NH)
1897.7.5:41-44 (when examined were numbered 42-44, 3 fish, 29.9-33.5 mm standard
length in poor condition, September 2007), with many others apparently in the Zoological
Institute, St. Petersburg (Eschmeyer et al., 1996).
Key characters
This species has a moderately slender body (17-22% of standard
length), a long and narrow head (interorbital width 13-15% of head
length), a sharply keeled belly, and rounded pectoral fin tips.
Morphology
Dorsal fin unbranched rays 3-4, usually 3, branched rays 13-15, and
anal fin unbranched rays 3, branched rays 14-21. There are 44-49,
usually 46-48, vertebrae, more than in the other two kilka species and
probably a consequence of the low water temperature larvae develop in.
Belly with 26-32 scutes. Gill rakers 42-51.
The bigeye kilka is adapted to life in deeper water having, as its
name indicates, big eyes with more rod cells and a weaker retina but
also more transparent body tissues than other kilkas.
Sexual dimorphism
None reported except size.
Colour
The back and top of the head are dark.
Size
Reaches 14.5 cm standard length.
Distribution
Found in the Caspian Sea and concentrated in the south including Iranian waters.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
The bigeye kilka is found further away from the coast than the
anchovy kilka at depths over 50-70 m, down to 450 m, with large
schools down to 130 m. It does not enter fresh water or low salinity
areas, staying well away from the shore. There is a daily vertical
migration, avoiding sunlight, and following food items. Larvae live in
water temperatures of 5°C.
Overwintering occurs in the southern Caspian at temperatures of 9-11°C,
a migration to the central Caspian takes place in spring, with a
return south in autumn (Prikhod'ko, 1979b).
Age and growth
Sexual maturity is attained usually at age 2, and 2-4 year olds
dominate catches, but life span is up to 8 years (Iranian Fisheries
Research and Training Organization Newsletter, 14:6, 1996). The
female is larger than the male at the same age. Growth is slower than
in C. engrauliformis. Males dominate the population (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996; Fazli et al., 2005) but this study may have sampled spawning fish (see below).
Fazli et al. (2005) examined fish from the main landing ports (Babolsar,
Amirabad and Anzali) found the mean fork length of fish increased from 95.87 mm
in 1997 to 105.0 mm in 2000 but then decreased to 102.3 mm afterwards. Over this
time period, fork length range became wider with specimens in the upper length
classes representing most of the catch. Six age classes were present, 1+ to 6+
years. During 1998-1999, age classes 1+ to 3+ comprised more than 90% of the
catch. In 2000, there was a decrease in age classes 1+ and 2+ and an increase in
3+ to 5+ classes. In 2001, age classes 3+ and 4+ decreased and classes 5+ and 6+
increased. The relative frequency of the bigeye kilka has decreased in recent
years as a result of the introduction of the ctenophore, Mnemiopsis leidyi,
a food competitor and predator on kilka eggs and young. Khorashadizadeh et al.
(2006) found fish in the Babolsar area of the Iranian coast to have 5 age
classes, dominated by the 4+ class. Fazli et al. (2009) examined changes
in the population biology of this kilka over the period 1995 to 2001, attributed
to the inavsive ctenophore. The overall sex ratio was 1.65:1 in favour of males,
length-weight regressions were W = 0.00922L2.851 for females and W=
0.008021L2.907 for males, indicating a negative growth for both
sexes, growth parameters were L∞ = 142 mm, K = 0.28 year-1,
and t0 = -1.39 years, the instantaneous coefficient of natural
mortality was 0.460 year-1, and the instantaneous coefficient of
fishing mortality varied between 0.469 and 0.980 year-1. Biomass
increased from 36,900 mt in 1995 to more than 53,500 mt in 1998 but declined to
less than 5900 mt in 2001. This was attributed to overfishing and the appearance
of the ctenophore, a competitor for zooplankton food.
Karimzadeh et al. (2010) examined
fish from the Babolsar region off Mazandaran and calculated growth parameters as
L∞ = 148.6 mm, K = 0.46/yr-1 and t0 =
-0.18/yr, instantaneous coefficient of natural mortality was 0.881/yr-1
and the current exploitation rate was estimated as 0.26.
Food
Migratory mysids often predominate in the planktonic diet of this
species. Fish fry are also eaten. Its foods are less diverse than that
of other kilkas because the variety is less in the deeper waters this
fish inhabits during the day. The three kilkas share the available
habitat and its foods, the common kilka in shallow, coastal waters,
the anchovy kilka in the upper layers of the open sea and the bigeye
kilka in deeper water of the open sea (Badalov, 1972; Prikhod'ko, 1979b).
Reproduction
Spawning is extended, from January through to September but is most
intense in spring and autumn (Prikhod'ko, 1979b). Males predominate in
the spawning areas, remaining there while females leave immediately
after spawning. Males are mainly at 10-20 m and females at 20-25 m
during the spawning season. Water temperatures at 6-13°C
and salinity 12.6-13.0‰. Fecundity is 28,300 eggs. In Iranian waters, mature
fish ready to spawn are always present in catches in winter and early spring
(Fazli et al., 2005).
Khorashadizadeh et al. (2006) found fish in the Babolsar area of the
Iranian coast to have peak spawning in early January.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean parasites Pseudopentagramma symmetrica (probably
Pronoprymna ventricosa after Youssefi et al. (2010)), Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum, Eustrongylides excisus,
and larvae of a Contracaecum and an Anisakis species (Iranian
Fisheries Research and Training Organization Newsletter, 11:4-5,
1996; Annual Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 28, 1997; Shamsi and Dalimi, 1996; Shamsi et al.,
1998; Shamsi et al., 1998).
Ghayoumi et al. (2009) found that fish from Babol Sar harbour contained
the intestinal helminths Corynosoma strumosum, Pronoprymna ventricosa,
Contracaecum sp. larvae and Anisakis sp. larvae, diet being the
main factor affecting diversity of the parasites. Varshoie et al. (2010) record the helminths Pseudopentagramma
symmetrica (see above), Bunocotyle cingulata and Mazocreas alosae in this
species from Iranian waters.
Clupeonella species are an important food fish for sturgeons
(59.4% by weight of sevryuga (Acipenser stellatus) diet in the Middle Caspian), Sander
(Percidae) and herrings and the Caspian seal. Predators consume 590
million kg of the three kilka species which themselves are the main
consumers of zooplankton. Kilkas are a very important element in the
life of the Caspian Sea (Badalov, 1972; Prikhod'ko, 1979b; Krylov,
1984). This species is taken to a lesser extent than other Clupeonella
species because it is relatively sparse.
Economic importance
The bigeye kilka catch amounts to about 70 million kg a year in
former Soviet waters of the Caspian by means of electric light. All
three kilka species are caught by using underwater electric lights and
fish pumps (Nikonorov, 1964) but in the case of the bigeye the effect
is avoidance used to drive it to the bottom where it can be caught.
Other kilkas are attracted to the light but the bigeye is a vertical
migrator, avoiding sunlight (Prikhod'ko, 1979b). Light-assisted
catches of kilkas damages young shad (Alosa) stocks which are
an incidental catch (Zakharyan and Teruni, 1979). Catches in Iranian
waters are only 6.84% of the total kilka take (Razavi Sayad, 1993b). The relative
frequency of the bigeye kilka in Iranian catches was ranked second after anchovy
kilka in 1990-1991 at 6.84%, increasing to 12.6% and 21.7% in 1997 and
1998 and then decreasing.
Omega-3 fatty acids from fish oil of this species has been tested as a
dietary supplement and was found to relieve symptoms of dysmenorrhoea (Moghadamnia
et al., 2010).
Conservation
Stocks in Iranian waters are said to be depleted. Kiabi et al.
(1999) consider this species to be of least concern in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, abundant in numbers, widespread range (75% of
water bodies), absent in other water bodies in Iran, and absent
outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Iranian material: CMNFI 1993-0167, 1, 93.0 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 2, 91.8-94.0 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E).
Genus Tenualosa
Fowler, 1934
This genus comprises 5 species found from the Indian Ocean to
Indonesia and China. A single species enters rivers of southern Iran.
The genus is defined by a series of characters listed below under Key
characters. These fishes form part of local, artisanal fisheries
throughout their range.
Tenualosa ilisha
(Hamilton, 1822)
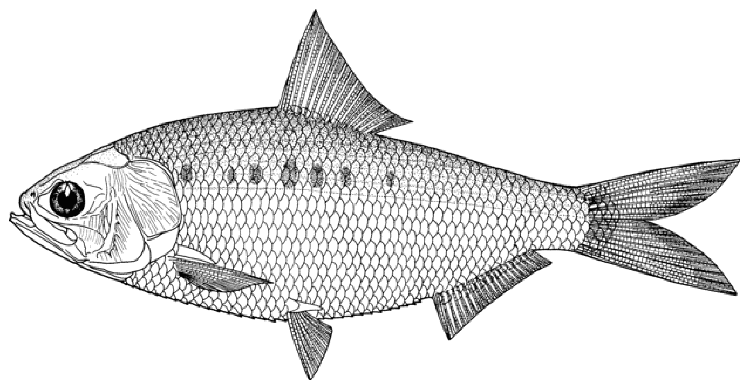
Common names
صبور (= sobur, soboor, sobour, sabur, zobur, zabur,
zamur or zomur, all variants of the same word), bari, barak;
mahi-ye khor kuchiku (= small bone fish, at Abadan from www.abadan.com/abadanhistory.html, 15 March 1998).
[zoboor, soboor, sbour in Arabic; hilsa, Indian shad or river shad;
palo, palla or pulla and tikki-palwar in Pakistan].
Systematics
Clupanodon ilisha was originally described from the Ganges
estuaries in India. Formerly placed in the genus Hilsa Regan,
1917. Al-Hassan (1982), citing a personal communication from a Mr. Al-Abaychi
in 1973, suggests that Shatt al Arab fish are distinct from those in
Pakistan on morphometric and meristic grounds but no data have been
published. Milton and Chenery (2001) used
genetic and otolith chemistry data that provided strong evidence for a distinct
stock in Kuwait, compared with stocks from India to Sumatra. Al-Hassan (1999) mentions that people in Basrah can
distinguish two kinds of sobur, based on taste. One is the tastier and
pricier Shatt al-Arab form and the other is the less desirable
estuarine/sea form. This has not been confirmed by systematic studies. Jorfi
et al. (2008; 2009) found differences between populations in Iran and Iraq using
molecular techniques.
Key characters
This species is distinguished from other Indian Ocean clupeids by
the upper jaw with a median notch, the anal fin ray count being less
than 30 rays, a terminal mouth (lower jaw not prominent nor flared at
the corners), scales in lateral series are not perforated posteriorly,
last dorsal fin ray not filamentous, weakly developed lines (the
fronto-parietal striae) on top of the head (usually covered by skin
and not visible), gill rakers on inner arches straight not curled, a
long head 28-32% of standard length, and 30-33 ventral scutes forming a keel
along the belly, 15-18 being prepelvic and 11-15
postpelvic (Al-Nasiri and Al-Mukhtar, 1988a, 1988b; Marammazi et al., 1995).
Morphology
Dorsal fin with 4-5 unbranched rays followed by 14-16 branched
rays, anal fin with 2-3 unbranched rays followed by 16-20 branched
rays, pectoral fin branched rays 12-15 and pelvic fin branched rays 7.
Lateral series scales 44-51. Gill rakers are fine and numerous, up to
about 275 on the lower arch.
Iranian fish examined by Marammazi et al. (1995) from the
Bahmanshir River in Khuzestan have 30-32 total scutes along the belly,
16-18 prepelvic scutes, 13-15 postpelvic scutes, 19-21 dorsal fin
rays, 19-24 anal fin rays, 13-15 pectoral fin rays, 8 pelvic fin rays
and 44-51 scales.
Sexual dimorphism
None reported.
Colour
The back is grey-blue, bluish to green and the sides are silvery
with golden, purplish or pink highlights. The dorsal fin is grey, the
caudal fin grey-blue with a silvery tinge and darkened margin, and the
anal fin is light blue with some silvery tinges. Paired fins are
hyaline. The area behind the gill cover in young fish and many adults
have a dark blotch followed by a series of spots or blotches running
along the upper flank, for a total of 6-7. The blotches may take the
form of bars. The eye is yellow to red. Young have a bronze back,
silvery flanks and a caudal fin margined in black.
Size
Attains 60.6 cm total length and 2.49 kg for females and 43 cm and
0.68 kg for males. A sample of 233 moribund fish from the Ashar Canal,
a branch of the Shatt al Arab, Iraq examined by Al-Nasiri and Al-Mukhtar
(1988a; 1988b) had a total length range of 70-152 mm. Hussain, Jabir
and Yousif (1994) record fish migrating to the Shatt
al Arab for breeding at 21-38 cm for males and 33-43 cm for females.
Mature females in the Shatt al Arab weighed about 0.5-1.1 kg (Jabir
and Faris, 1989). Fishes from Kuwait attained 57 cm (Al-Baz and Grove, 1995).
Fishes from the Arvand, Bahmanshir, Karun and Dez
rivers of Iran were 120-500 mm long (Marammazi et al., 1998; Ghafleh
Marammazi et al., 2004).
Distribution
Reportedly found from the Red Sea and Persian Gulf through the
Indian subcontinent to the Malayan Archipelago in some general works,
or more narrowly from the Persian Gulf to Myanmar. It enters the Shatt al
Arab and Tigris River, once as far north as Baghdad (Kanazawa, 1955),
but the northernmost distribution today in Iraq is the Hawr al Hammar.
Before the construction of dams on the Euphrates the migration was up
to "Yaou" and "Meshkhau" and up to Qal`at Salih
(31°31'N, 47°16'E) in the Tigris of Iraq (van den Eelaart, 1954).
The lower reaches of the Tigris and Euphrates rivers were connected
by a channel to the Khor Al-Zubair in Iraq during 1983. As a
consequence the Khor became oligohaline (at less than 10‰) rather
than hypersaline (at more than 40‰), becoming an estuary with heavy
reed growth. The catch of sobour in the Khor by 1997 exceeded that in
the Shatt al Arab and may involve diversion of stocks from the
original habitat of the Shatt (Hussain, 1997).
In Iran, it is recorded as far north as
the Gargar Shoteit on the Dez River (Marammazi, 1994). Hussain,
Jabir and Yousif (in litt., 1995) record this species from the Shatt al
Arab in Iraq and the Bahmanshir, Jarrahi, Zohreh and Hilleh
rivers in Iran. Marammazi (1994) and Marammazi et al. (1998) report this
species from the Arvand, Bahmanshir, Karun and Dez rivers. Ghafleh Marammazi
et al. (2004) record it from the Zohreh, Bahmanshir, Arvand and Karun rivers in Iran.
It may be found in the Hormuz basin but this has not been verified with
specimens.
In the sea, they are found from Bushehr around to
Kuwait in coastal waters (Blegvad and Løppenthin, 1944; Hussain,
Jabir and Yousif, in litt., 1995).
Zoogeography
Al-Hassan (1982) mentions a study comparing a population of this
species from Basrah, Iraq with one from Pakistan and finding
significant meristic and morphometric differences, perhaps indicative
of distinct stocks.
Habitat
Sobour enter the Shatt al Arab in February and March during high
tides and feed there until the fall according to a study by Al-Nasiri
and Al-Mukhtar (1988a; 1988b) working on fish taken from the Ashar
Canal, Basrah, Iraq. van den Eelaart (1954) reports that most fish
enter the Shatt al Arab in April during the last and first phase of
the moon and anecdotal reports indicate the end of March to be the
peak period of entry. They ascended into the Hawr all Hammar and
from there into the Euphrates as well as into the Tigris (van den Eelaart,
1954). Significant numbers were recording as entering the recovering Hawr al Hammar in 2005-2006
(Hussain et al., 2006). Small specimens (50-100 mm) were observed in the east Hawr al
Hammar in June 2005 and July 2006 (www.iraqmarshes.org, downloaded 29 August
2005; N. A. Hussain, in litt., 2006). In mid-April sbour were found below
the Yaou and Moshkhab regulators which formed the limit of their migration on
the Euphrates in the early 1950s. The limit in the Tigris was beyond Amara. The
main spawning grounds in the Euphrates were probably somewhere between Shinafiya
and Samawa and in the Tigris between Amara and Qalat Saleh.
The last ones leave the Shatt in July and fry
are found in the rivers of Iraq at the end of the June. Hussain, Jabir
and Yousif (1994) record sobour ascending the Shatt
al Arab during March with a continuing migration upstream through
April to July for spawning and a return migration to the sea during
August to October. Al-Hassan (1993) notes that local people believe that sobour
ascend the Shatt al Arab during spring to marshes north of Basrah for spawning,
suggesting that they are the fluvial anadromous type. Al-Hassan (1999) considers they migrate to the sea
in September-November, when they are landed in Kuwait, and they then
migrate to the Iranian coast during December-January. Males and
females move upriver in separate groups according to Iraqi fishermen (Al-Hassan, 1999).
Jorfi
et al. (2008) suggest, based on molecular studies, that a population in the
Persian Gulf chooses the Karun River for spawning and migrates via the
Bahmanshir River, while others migrate up the Tigris and Euphrates rivers in
Iraq via both the Bahmanshir and the Arvand rivers.
Blegvad and Løppenthin (1944) mention this species on sale at
Khorramshahr on 28-29 April. The spawning migration in Iran occurs in
spring (I. Sharifpour, in litt., 1991). It is only found in the
Zohreh River in spring and summer (Marammazi, 1994).
They may be found in deep water, over 18 m, or in shallows, on
their spawning migration. Large concentrations of sobour occur below
dams blocking their migration. Young occur in side branches of the
Shatt al Arab near food, shelter and the spawning grounds (Hussain,
Jabir and Yousif, in litt., 1995).
This species occurs in river estuaries and coastal waters and
appears to be restricted to the northern end of the Persian Gulf
because this is the only part with large spawning rivers (Hussain,
Jabir and Yousif, in litt., 1995). These authors also suggest
that an anadromous stock from the Shatt al Arab migrates to warmer
waters off Bushehr during January, February and March. At the same
time there is a winter decline of Kuwaiti stocks. There may also be a
marine stock inhabiting coastal waters of Kuwait since larvae have
been found in Kuwait Bay during June and November and catches are made
in the Bay year round.
Biogenic and anthropogenic sources were noted for the hydrocarbons in this
species from the Shatt al Arab; n-alkanes attained 31.11 µg/g and hydrocarbons
10.91 µg/g, the highest for the fish species studied (Al-Saad et al.,
1997). The fat content of this shad is a factor in these high levels (Al-Saad,
1990).
Hussain (1997) notes that the changing conditions in the Khawr az Zubayr,
which became oligohaline from hypersaline after it was connected to the
Tigris-Euphrates basin by the Shatt al Basrah Canal. In 1994 fishermen began
catching sbour in the Khawr az Zubayr and by 1997 the numbers caught exceeded the
catch in the Shatt al Arab.
Migrations in the Indus River of Pakistan (Islam and Talbot, 1968)
may last over 7 months and the migration up the Ganges River extends
over 1287 km. Fish may move as much as 70.8 km in one day and may jump
out of the water on the migration.
Age and growth
In the Bahmanshir River, Iran most fish are 4-5 years old. The
minimum total length and age at maturity are 26.2 cm, 200 g and 2
years for males and 32.18 cm, 450 g and 3 years for females. Von
Bertalanffy growth parameters in Iranian females are L∞
= 57.78 cm and K = 0.282 and in males 46.37 cm and 0.252 (Marammazi,
1995; Iranian Fisheries Research and Training Organization
Newsletter, 12:5, 1996; Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 53-54, 1997).
Hashemi et al. (2009) studied fish landed at Hendigan and Abadan and
recorded L∞ as 42.81 cm, K was 0.9, M
was 1.37, F was 2.41, Z was 3.78 and E was 0.64. Y'/R was 0.048 and B'/R was
0.19, exploitation rate (U) was 0.61, annual stock at the beginning of the year
(P) was 7615 t, annual standing stock (b) was 1927 t and MSY was 3642 t. The
stock was overfished.
Hashemi et al. (2010) studied 9317 fish from landings at Abadan and
Hendijan. Size range was 20-39 cm. The von Bertalanffy growth parameters were L∞
= 43.32 cm, K = 0.78 yr-1, Φ' was 3.16 and t0 was -0.18.
Mortality rates were M = 1.29 and Z = 4.53, and fishing mortality (F) was 3.24
yr-1. The exploitation rate (E) was 0.72 and the stock was overfished.
Values of the sizes where the probability of capture was 50% (L50)
and 100% (L100) were 22.3 and 28.5 cm TL respectively. Fish
were recruited to the fishery at a mean size of L100 =
22.3 cm. The relative yield per recruit (Y'/R) was 0.062, relative biomass per
recruit (B'/R) was 0.12 and exploitation rate (U) was 0.76. The values for
annual catch, total annual stock, standing stock and maximum sustainable yield
were 4645 t, 6635.71 t, 1433.64 t and 3274.19 t respectively. The fishing
pressure must be reduced from 3.24 yr-1 to about 0.97 yr-1for
this population to be adequately managed. Another study apparently based on the
same or similar samples (Hashemi Seyed et al., 2010) found slightly
different parameters: L∞
= 42.81 cm, K = 0.0 yr-1, t0 = -0.25. Z = 3.78, M = 1.37,
F = 2.41, E = 0.64 and the values for annual catch, annual average standing
stock and maximum sustainable yield were 7615 t, 1927 t, and 3624 t
respectively.
Roomiani and Jamili (2011) examined fish
landed in Iran from a northern Persian Gulf fishery. Growth was isometric.
Maximum total length was 43 cm and weight 949 g. von Bertalanffy growth
parameters were L∞ = 42.74 cm total length, K = 0.77 and
t0 = -0.21 years-1. Total mortality (Z) was 2.55 years-1,
natural mortality was 0.75 years-1, fishing mortality was 1.8 years-1,
and exploitation rate (E) was 0.7 years-1, and parameters indicate
overfishing. Maximum sustainable yield was calculated to be 2653 t.
Al-Nasiri and Al-Mukhtar (1988a; 1988b) give a length-weight relationship of
W = 3.9 x 10-6 L3.16 or log W = 3.16 log L-5.4 for fish
aged at 0+ from the Ashar Canal at Basrah. The mean condition factor was 0.87.
Fishes in the Shatt al Arab are in age groups 5 to 6 for the period May to
August (Hussain et al., 1991). In contrast, a later study on the Shatt al
Arab fish showed there are 5 age groups and the second and third age groups
dominate in catches (Hussain, Jabir and Yousif, 1994). In this latter study,
Shatt al Arab fish mature at 25 cm for males and 33 cm for females, similar to
an Iranian study (see below). The length-weight relationship was log W = -4.7074
+ 3.0479 log L for females and log W = -4.5802 + 3.0193 log L. Condition factor
gradually increased with length groups in males, peaking at 32-33 cm followed by
a sharp decline while females had a nearly stable condition factor from 34 to 43
cm. Mohammed et al. (2001) gave a von Bertalanffy growth equation as L∞
= 60.47 cm and a condition factor of 0.32, slower growth than in Indian and
Bangladesh populations and probably maturing later.
Amodeo (1956) gives lengths of 25 to 35 cm for fish caught in the Shatt al
Arab on their spawning migration. Young grow rapidly, 4.3 cm in
October-November. Most fish on the migration in the Indus River were in age
groups 3 and 4. Life span is up to an estimated 7 years with maturity as early
as 1 year. Jawad et al. (2004) found haematocrit level to increase with
body length up to 40 cm after which it decreased, males showed higher levels
than females, and levels were higher pre-spawning than during spawning and
increased slightly post-spawning, a general correlation with fish activity.
Al-Baz and Grove (1995) studied fish taken from Kuwait fish markets. Females
dominated the catch, male:female ratio being 1:2.4, perhaps because the sexes
moved in different schools. The smallest mature female was 34.4 cm and 50% of
the females are mature at 41.5 cm. They estimated natural mortality (M) based on
von Bertalanffy growth parameters (L∞ and K) and mean annual water
temperature as log M = -0.0066 -0.279 log L∞ + 0.6543 log K + 0.4634
log T. The length-weight relationship was W = 0.011 L2.983 for males
and W = 0.007 L3.104 for females. Growth in the sexes follows
different patterns. Five age groups were detected using otoliths and fish were
fully recruited to the fishery at 3 years of age. von Bertalanffy growth
parameters were L∞ = 52.70 cm and condition factor (K) = 0.28 per
year while using Allen's method they were L∞ =52.50 cm and condition
factor (K) = 0.36 per year Growth curves were given. Annual total mortality was
estimated to be 1.2 using the K value of 0.36. A fishing mortality was
calculated to be 0.8 per year.
Food
The Ashar Canal study found them to feed on phytoplankton such as
dinoflagellates and diatoms and on zooplankton, mainly copepods, as
well as their own young. The sieve-like gill rakers are used to strain
out planktonic organisms without selection. Presence of some sand
grains indicates that feeding can occur on the river bed. Feeding
intensity may decrease or cease on the spawning migration and is very
high after spawning. The Bahmanshir fish feed principally on copepods
and diatoms. Shatt al Arab juveniles feed mostly on filamentous algae
and diatoms with some organic matter, fish eggs and zooplankton while
adults have empty stomachs on the spawning migration (Hussain, Jabir
and Yousif, in litt., 1995). In the Indus River, the newly hatched
larvae and juveniles graze for five to six months in fresh waters before they
migrate to the sea (www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm, downloaded 18 February 2003).
The prime food in the sea off the Iranian coast is phytoplankton, principally
Bacilliariophyta followed by Pyrrophyta. Zooplankton is also taken (Nasri Tajan
et al., 2008).
Reproduction
The spawning migration depends on the flood regime of the rivers. Turbid
water and fast current are probably stimulants to egg deposition. The sbour
depends on river-edge vegetation for egg deposition. Spawning grounds in Iraq
are probably located near the beginning of the side branches of the northern
sector of the Shatt al Arab, 120 km from the sea (Hussain, Jabir and Yousif,
1994). This species is gonochoristic (Blaber et al.,
1997). Males may ascend the river before females but females become
dominant in Indian populations. Males dominate in March in the Shatt
al Arab and the sex ratio reaches equilibrium in the spawning months
of May-July (elsewhere in the same communication spawning is given as
June to August) (Hussain, Jabir and Yousif, 1994; Jawad et al. (2004).
Spawning may occur more than once in a season in India. This has not
been demonstrated for Iran but could occur. The gonadosomatic index
for fishes in the Iraqi Shatt al Arab indicates peaks in March-May and
July-August, suggesting two spawnings (Hussain et al., 1991)
although a later report (Hussain, Jabir and Yousif,
1994) gives spawning as June to July and July to August as evidenced by two
modes of juveniles found in September. Sex ratio is equal during this period.
All females entering the Shatt al Arab were mature with smallest female being 33.0 cm long. Males less
than 25.0 cm were immature, the population reaching 100% maturity at 31-32 cm
(Hussain, Jabir and Yousif, 1994). The Kuwait fish studied by Al-Baz and Grove
(1995) indicated spawning between May and July with a peak in June.
Fecundity in the Indus River population was estimated to be up to
2,917,000 eggs per female, egg diameters reached 0.89 mm, and the hatching takes
place in about 23 to 26 hours (http://www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm,
downloaded 18 February 2003). Estimates for the Hooghly River of India reach 13,230,500 eggs per female (Al-Hassan, 1993). Fecundity
in the Shatt al Arab ranges between 444,960 and 1,616,560 eggs for
fish 33.0-41.5 cm total length although 2 fish 37.3 and 2 fish 39.0 cm
total length had a range in egg numbers of 109,000-233,840, showing
that great variations in fecundity occur between individuals; possibly
some fish had partially spawned before capture (Jabir and Faris, 1989). This
latter study gave a relationship between absolute fecundity and total length as
F = 1.3699 L3.6681 and log F =
0.1367 + 3.6681 log L and between fecundity and weight F = 302.8214 W1.2087
and log F = 2.4812 + 1.2087 log W. Fecundity increased significantly with body
weight, ovary weight and total length. Relative fecundity (ova/gramme body
weight) varied from 737 to 1721, mean 1216.
Hatching can occur within one day at an average temperature of 23°C.
Eggs, larvae and young are found on the spawning grounds but with
growth the young move into estuarine and foreshore areas during winter
months. Hussain, Jabir and Yousif (1994) record the
appearance of juveniles from the northern Shatt al Arab from June to
November. Adults return to their original habitat in the sea after
spawning. There is some evidence for freshwater resident populations
in India which migrate upriver to spawn but do not descend to the sea.
The Bahmanshir fish are thought to spawn from April to July. Only
adults enter the Bahmanshir (Iranian Fisheries Research and
Training Organization Newsletter, 12:5, 1996). Absolute fecundity
of fish from the Arvand, Bahmanshir, Karun and Dez rivers ranges from
374,892 to 1,954,144 eggs for total lengths of 380 to 500 mm
respectively and is related to age. Ova with diameters 0.64-0.795 mm were
released spontaneously in a study of this fish in Khuzestan province, in several
batches along its migration route (Ghafleh Marammazi et al., 2004).
Spawning begins on entry to the Bahmanshir and Arvand rivers in Khuzestan in
April, continuing to September and the end of their migration at the cities of
Shushtar and Dezful higher upriver. Males enter these rivers first in March,
followed by females in April (Ghafleh Marammazi et al., 2004).
Parasites and predators
None reported from Iran other than nematode larvae by Ebrahimzadeh and Nabawi (1975) for fish from the Karun River.
Economic importance
The Ashar Canal study cites 996,308 kg reaching the Ashar fish
market from October 1975 to June 1977 (see also Sharma, 1980). The
catch landed at Fao on the Shatt al Arab estuary of Iraq was 6576
t in 1990-1991 (L. A. J. Al-Hassan, in litt., 1995;
however this seems much too high although the estimate is from the
Food and Agriculture Organization). This species forms the most
important commercial fishery in the Basrah region of southern Iraq,
average catches being 491.086, 319.661 and 267.988 t in 1977, 1978
and 1979 respectively (sic, Jabir and Faris, 1989). There is a drift-net
and stake-net ("hadra") fishery in the sea by Kuwait
in Kuwait Bay and around Falaikah Island (Al-Baz and Grove, 1995).
The fishing season on the Tigris-Euphrates is March to August with a peak in
April, or late April to early June (Jabir and Faris, 1989) or to November (Ali
et al., 1998). van den Eelaart (1954) gave the fishing season for this species as
March-August (peaking in April) in rivers, and March-May (peaking in April) in
Hawr al Hammar, Iraq. Fish are caught at the mouth of the Shatt al Arab as they
enter the river with stationary gill nets, drifting gill nets, in "mailan" and
"odda" traps from March to August. The catch averaged 150-180 kg per ten odda
and in March 1953 the total catch at the mouth of the Shatt al Arab was about
25,000 kg (Amodeo, 1956). Large fish are only caught in the summer (Al-Hassan,
1999).
The catch at Abadan from February to November in 1943 was about 401.42
t and from January to June about 336.67 t (Pillay and Rosa, 1963).
This species is seen on markets at Ahvaz, Khuzestan in November but these are
sea-caught fish. Marjan Iran Company was selling 600-800 g fish for
U.S.$1.40/kg, 800-1000 g fish for U.S.$1.60/kg, 1000-1200 g fish for
U.S.$1.70/kg, and 1200 g and larger fish for U.S.$1.80/kg in August 2003
(http://groups.yahoo.com/groups/hilsa/message/25). The catch in Khuzestan
province in 2000 was 2688 t (Ghafleh Marammazi et al., 2004) and in
2006 was 4989.83 t (about 15% of Khuzestan's total commercial fish landing) (Roomiani
and Jamili, 2011). The catch in Khuzestan Province in 2008 was 4645 t (Hashemi
et al., 2010).
These fish are caught with traps, weirs, gill nets and other
devices in rivers on the spawning migration. They are excellent eating
until spawning occurs after which they lose their flavour. However
this species has been implicated in clupeotoxic poisoning. Hindi et al. (1996a) give the chemical composition of flesh of this
species as 66.41% moisture, 12.12% fat, 18.72% protein and 1.98% ash, indicating
a valuable food fish characterised as fatty. Hindi et al. (1996b) give
chemical indices for assessing fish freshness according to the month of capture
and marketing (pH 6.06, total volatile nitrogen bases 15.32 mgN/100g fish,
thiobarbituric acid 1.35 mg, and free fatty acids 1.33%). Salari and Sadough
(2009) compared heavy metal (Cd, Pb, Cu, Co, Ni) content in muscle, liver and
gill tissues of fish from the Karun River and found levels less than those
considered dangerous in Iran.
In Pakistan, the Indus River fishermen number between 8,000 and 9,000. Jafri
(1994) reviews the Indus fishery which had yields up to 2694 mt. It is the most important Indo-Pacific shad species.
The failure of the Indus River fishery in 2003 through drought resulted in
Iranian fish being flown to Pakistan for marketing there at rupees150-400 per
piece (www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm, downloaded 18 February 2003).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, in aquaculture and in textbooks.
Conservation
Hussain, Jabir and Yousif (in litt., 1995) report a decline
in catches over the previous two decades in the Shatt al Arab. Al-Nasiri and Al-Mukhtar (1988a;
1988b) mention that fish enter the polluted Ashar Canal, a side tributary of the
Shatt al Arab, during high tide when waters are diluted. A low tide in October
resulted in severe oxygen depletion and fish suffocated. Das et al.
(1977) found samples from the Ashar fish market in Basrah to be contaminated
with hydrocarbons, emitting a kerosene smell and being unfit for human
consumption. Al-Saad (1990) found petroleum hydrocarbon residues to be high in
Khawr az Zubayr fish at 40.6 μg/g as this species is one that accumulates fat.
Evidently, overfishing and pollution are major factors in the conservation of
this species, to which must be added variations in freshwater flow and quality
from the marshes and Tigris-Euphrates through human processes.
Further work
The migratory habits and ecological requirements of this food fish
need to be examined in more detail for Iranian waters.
Sources
Some aspects of the biology of this species were based on Pillay
and Rosa (1963) and Al-Hassan (1993) writing mostly on Indian and Pakistani populations. Specimens
on markets in Ahvaz, Khuzestan examined.
Iranian material: CMNFI 1991-0153, 1, 243.3 mm standard length, Khuzestan, Zohreh River (no other locality data).
Comparative material: BM(NH) 1875.1.14:11-13, 3, 118.8-135.8 mm standard length, Iraq, Tigris River (no other locality data);
BM(NH) 1920.3.3:178-182, 6, 103.3-132.4 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1989.1.13:1-3, 3, 53.9-59.9 mm standard length, Iraq, Khawr az Zubayr (no other locality data);
BM(NH) 1989.1.13:4-5, 2, 66.6-69.8 mm standard length, Iraq, Khawr az Zubayr (no other locality data).
© Brian W. Coad (www.briancoad.com)






.jpg)















